Cancer Metabolism
Cancer metabolism is complex and still unclear as there is a lot of biological and cellular diversity in tumors and blood borne cancers. Genetic mutations, transcription networks, influence of microbiome, human diversity makes understanding cancer biology and cancer treatments difficult. At HMT we can provide quantitative metabolomics of the aerobic and anaerobic respiration, glucose oxidation and ATP synthesis using one of several different platforms. Our C-SCOPE platform focuses on Warburg effect, Urea cycle, purine metabolism, while our OMEGA scan platform is more inclusive covering additional pathways for fatty acid oxidation and carnitine transport. Our project scientist will help provide detailed pathway maps using natural or heavy isotope tracking.
<
Download Cancer Metabolism Pathway
Cancer Cells
In addition to this addiction to glucose, cancer cells rely heavily on glutamine as both a source of carbon anaplerosis as well as a nitrogen source for purine and pyrimidine synthesis. As cancer cells are studied in light of their metabolic needs, additional pathways are uncovered that play pivotal roles in the growth and maintenance of the tumor. These pathways include serine/glycine synthesis, choline synthesis, and glutathione production.
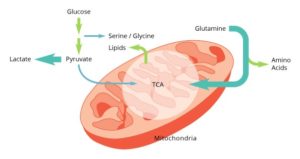 Targeting a cancer cell’s errant metabolism holds the promise of providing a more personalized approach to cancer therapy. Treatments can be designed specifically for the tumor cells, leaving normal cells virtually unharmed. Harnessing the power of metabolomics will uncover a tumor cell’s unique metabolic program leading to more effective therapeutic targets.
Targeting a cancer cell’s errant metabolism holds the promise of providing a more personalized approach to cancer therapy. Treatments can be designed specifically for the tumor cells, leaving normal cells virtually unharmed. Harnessing the power of metabolomics will uncover a tumor cell’s unique metabolic program leading to more effective therapeutic targets.
Today’s research is focused on how cancer cells produce the energy that is needed for proliferation and survival, how essential nutrients such as glucose and glutamine are utilized by the cells, and which metabolic pathways are essential to survival and therefore potential therapeutic targets.
Whether your target is metabolic or based on a signaling cascade, cell metabolism is likely to be affected. Cellular energy imbalance and oxidative stress are important indicators of cell viability and response to therapeutic intervention. Metabolic changes may even underlie drug resistance or sensitivity.
Cell metabolism can be viewed as a key regulator of cell health and survival and its understanding is therefore paramount to developing effective and specific therapeutics.
Application Examples
- SHMT2 drives glioma cell survival in ischaemia but imposes a dependence on glycine clearance
- Arginine Deprivation Inhibits the Warburg Effect and Upregulates Glutamine Anaplerosis and Serine Biosynthesis in ASS1-Deficient Cancers
- Subpopulation targeting of pyruvate dehydrogenase and GLUT1 decouples
- metabolic heterogeneity during collective cancer cell invasion
- Targeting pheochromocytoma/paraganglioma with polyamine inhibitors
