Alanine
Alanine is a neutral amino acid and an essential building block for protein synthesis, along with Leucine. Alanine is one of the two most common protein amino acids. Alanine is also of the most common plasma biomarkers for diseases. Alanine is not a dietary essential amino acid, as it can be made within cells.
How is alanine so prevalent as a disease biomarker and in dysregulated in many cell studies? What could be its role as a biomarker and what pathways other than protein synthesis?
- Alanine is a zwitterion, consequently it can act as an osmoprotectant under cellular stress and act as a protein stabilizer.
- Alanine comes in two stereosiomers, D & L, that activate sweet taste receptors, however, L-Alanine with high levels of nucleotides contributes to Umami taste while D-Alanine contributes only sweet.
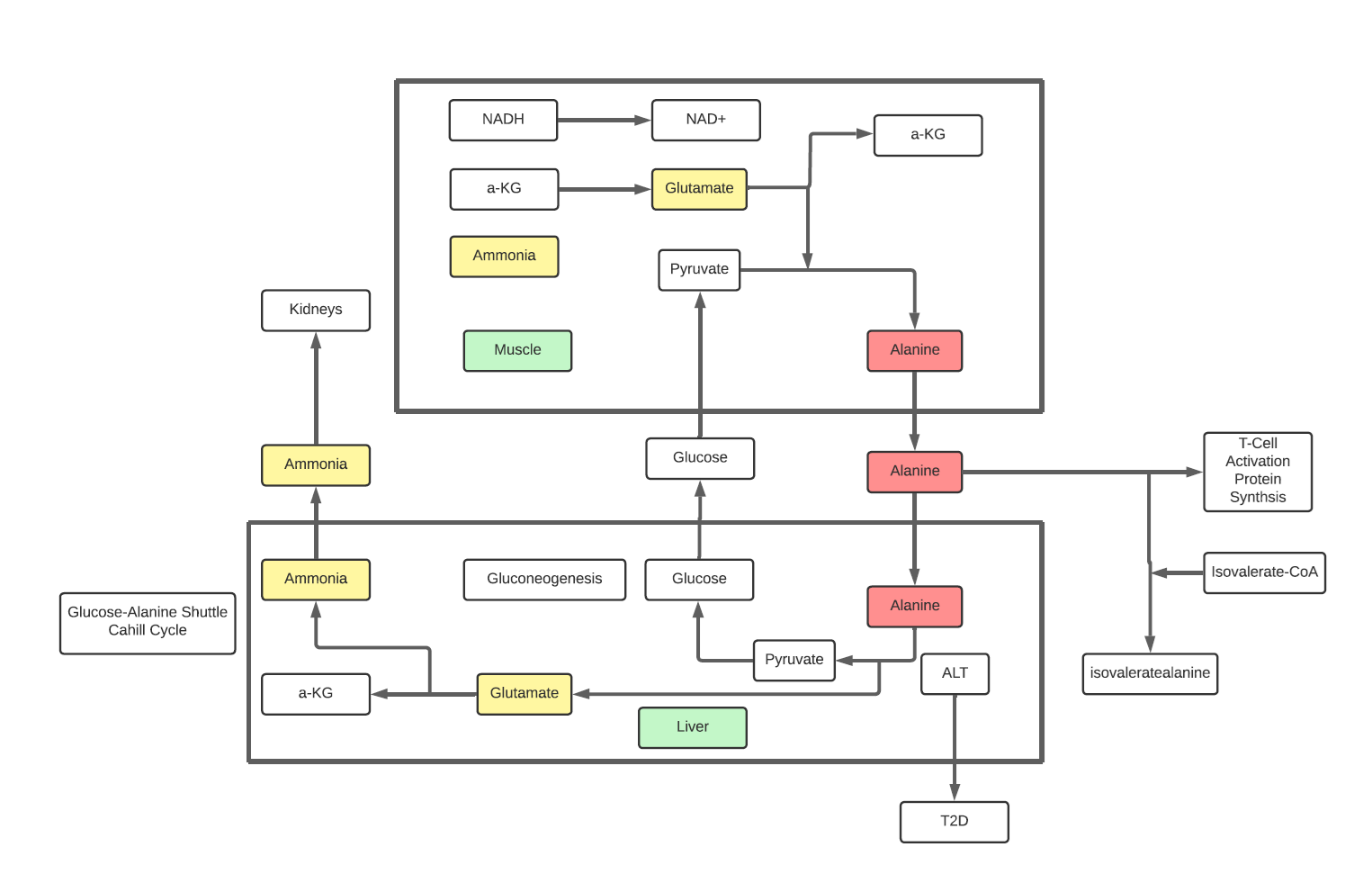
- Alanine, released from skeletal muscle, is used as a substrate for gluconeogenesis in the liver. A liver enzyme, Alanine aminotransferase(ALT), catalyzes the reaction to form pyruvate from alanine in the liver, pyruvate converting to glucose back in muscle (which is known as the “glucose-alanine cycle”. Hence Alanine plays a critical role in muscle metabolism
- There is more to this story than just Alanine to glucose. ALT catalyzes the transfer of an amino group from alanine to alpha-ketoglutarate in the alanine cycle to form pyruvate but there is a second product, glutamate, a critical amino acid itself and part of the transport of ammonia from muscle to liver to kidney.
- Lymphocytes depend on the import of extracellular alanine as vital for the transition from quiescence to activation of both naïve and memory T cells. Though the role is under study, the mechanism of which is not clear, but gluconeogenesis may be one factor to active cells as well as protein synthesis.
- Alanine racemase is a bacterial enzyme that catalyzes the conversion of L-alanine to D-alanine and is dependent upon Vit B6. This function is critical for the growth of bacteria due to their need for D-alanine as an essential component in the biosynthesis of cell wall peptidoglycan in both gram-positive and gram-negative bacteria thus important for supporting our microbiome.
- Another role for D-alanine is through D-amino acid oxidase (DAO) which catalyzes the oxidation of bacterial D-amino acids, such as D-alanine, and generates hydrogen peroxide, which is linked to antimicrobial activity. D-alanine is made exclusively within the microbiome, where as, DAO is conserved widely in eukaryotes, but not in bacteria hence DAO is considered a potential component of the innate defense in mammals.
- The dipeptide, Ala-Ala, is commonly observed in human blood and believed to be from beef, has a sweet taste and has shown anti-hypertensive activity against ACE-I.
- Alanine can be appropriately referred to as alpha-alanine as compared to beta-alanine, which has a different biochemical profile.
Arginine
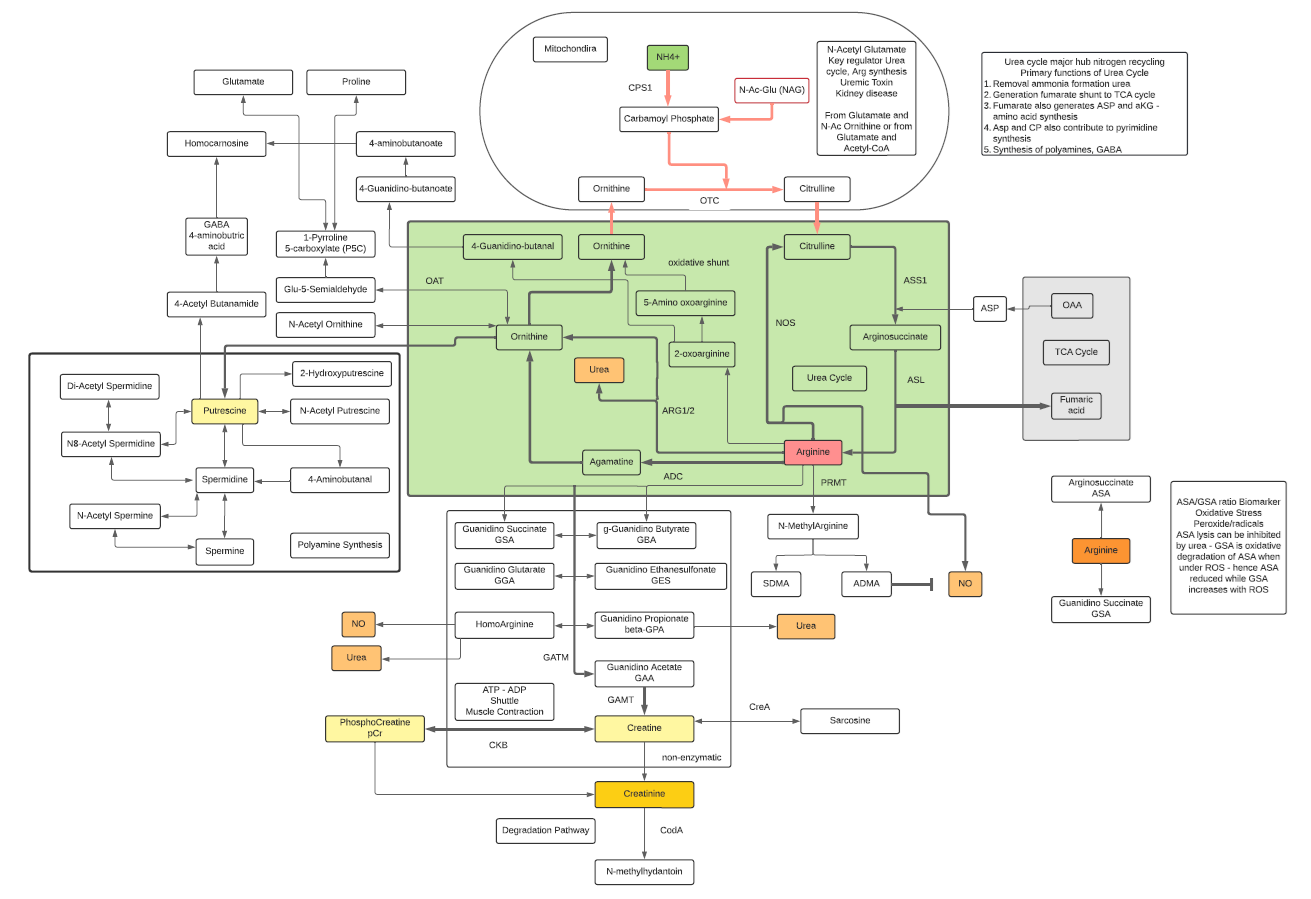
- L-Arginine participates in major pathways such as the Urea Cycle and Nitric Oxide synthesis.
- L-Arginine acts as a vasodilator, opening (dilating) blood vessels. Many people take oral L-arginine to treat heart conditions or for erectile dysfunction. This action presumably is through generation of nitric oxide (NO) from the conversion of Arginine to Citrulline.
- L-Arginine is classified as a basic amino acid. It is classified as a semi-essential or conditionally essential amino acid, depending on the developmental stage and health status of the individual. Hence, while there are dietary requirements, Arginine can also be synthesized within cells. Adults can synthesize Arginine in the urea cycle as part of the disposal of waste ammonia.
- L-Arginine has a bitter taste.
- L-Arginine plays an important role in immunity and wound healing through NO generation and transcription activation.
- L-Arginine is necessary for the synthesis of creatine and phosphocreatine (ATP recycling) and can be used for the synthesis of polyamines through transformation to Ornithine.
- The conversion to asymmetric dimethylarginine (ADMA) inhibits the nitric oxide synthase (NOS) reaction, providing negative feedback control. ADMA is considered a marker for vascular disease as high levels can inhibit NOS.
- L-Arginine stimulates the release of hormones; specifically, growth hormones and prolactin.
- L-Arginine is a known inducer of mTOR and is responsible for inducing protein synthesis through the mTOR pathway.
- disease states such as sepsis, injury, and cancer can cause an increase in L-Arginine utilization, which can exceed normal body production, leading to L-Arginine depletion.
- L-Arginine activates AMPK which then stimulates skeletal muscle fatty acid oxidation and muscle glucose uptake, thereby increasing insulin secretion by pancreatic beta-cells.
Cysteine
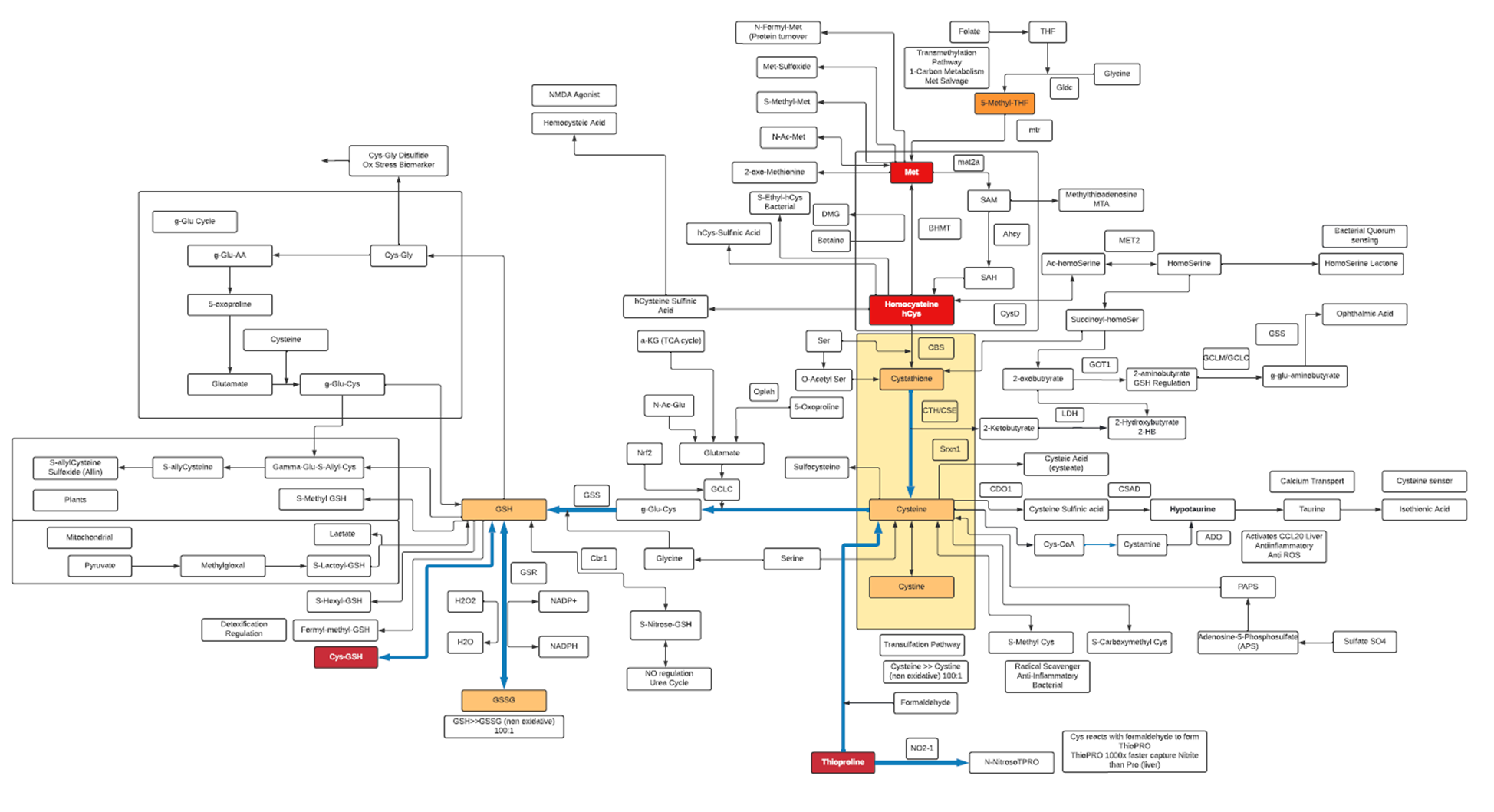
- Aside from being an essential building block for protein, L-Cysteine is also a component of the strong antioxidant Glutathione (GSH), which also serves as a reservoir of L-cysteine.
- The liver addresses both the need to have adequate L-Cysteine to support normal metabolism and the need to keep L-Cysteine levels below the threshold of toxicity.
- L-Cysteine has antioxidant properties on its own, while D-Cysteine is known to be toxic to bacteria.
- L-Cysteine is oxidized to a dimer, Cystine, which is readily transported into mammalian cells. In cells, Cystine is reduced back to L-Cysteine, which is an essential substrate for the synthesis of biomolecules such as proteins, Coenzyme A and for mTORC1 signaling.
- L-Cysteine is classified as a non-essential amino acid. Mammalian cells utilize the trans-sulphuration pathway for the synthesis of L-Cysteine from L-Methionine, most of which occurs in the liver.
- The L-Cysteine moiety of GSH can be liberated via γ-glutamyl cycle in which exported GSH is cleaved sequentially by γ-Glutamyl Transpeptidase (GGT) and DiPeptidases (DP) to release L-Cysteine which is then imported into the cells.
- The first and rate-limiting step of GSH synthesis is catalyzed by Glutamate-Cysteine Ligase (GCL), which is regulated by L-Cysteine availability at the transcriptional and translational level.
- The thiol functionality in L-Cysteine can undergo oxidation/reduction (redox) reactions. It can also undergo nucleophile addition and substitution reactions with oxygen, methyl transfer and acylation producing metabolites such as L-Cysteic acid, Lactoyl-L-Cysteine, S-Methyl L-Cysteine and many others. These metabolites may act as reservoirs for L-Cysteine or detoxification end products or oxidative products, and as such can be biomarkers for changes in oxidation state, transulfation and methylation pathways
- N-Acetyl-L-cysteine (NAC) is a powerful antioxidant. As a drug, NAC is used by healthcare providers to treat acetaminophen (Tylenol) poisoning.
Aspartic Acid
- L-Aspartic acid is the Swiss army knife of amino acids, being versatile and participating in a variety of critical roles.
- D-Aspartic acid is one of only 2 amino acids (the other one Serine) that exists as a common D-amino acid.
- The D form, D-Aspartic acid, is linked to neurotransmission, steroid hormones and appears to be regulated.
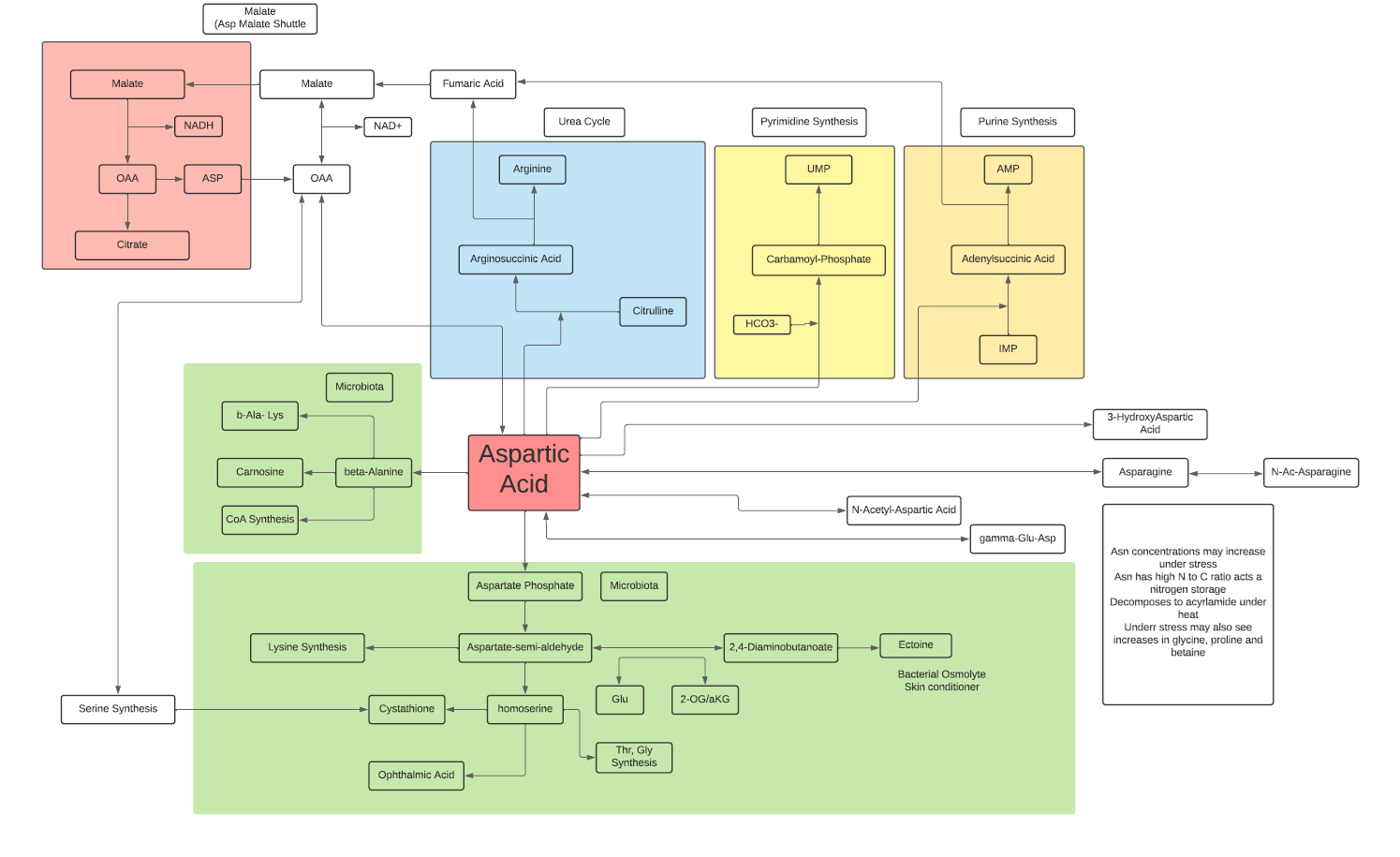
- L-Aspartate participates in the Malate Aspartate Shuttle to provide additional NADH from the cytosol to the electron transport chain in mitochondrial matrix.
- L-Aspartate reacts with L-Citrulline leading to L-Arginine synthesis and the release of Fumarate to TCA cycle.
- L-Aspartate reacts with bicarbonate leading to synthesis of UMP.
- L-Aspartate combines with IMP to lead to synthesis of AMP.
- L-Aspartate can act as a neurotransmitter and immunostimulant.
- Both L- and D- Aspartate activate the sweet receptor, however, when present with nucleic acids, L Aspartate can contribute to a UMAMI taste response.
- L-Aspartate is a non-essential amino acid in that it is synthesized mainly from OAA in the cytosol, but also can be generated from glutamic acid with Vit B6 as co-factor.
- Bacteria can use L-Aspartic acid to generate beta-Alanine leading to CoA synthesis and carnosine.
- Bacteria can use L-Aspartic acid to lead to synthesis of several amino acids, homoserine and ectoine.
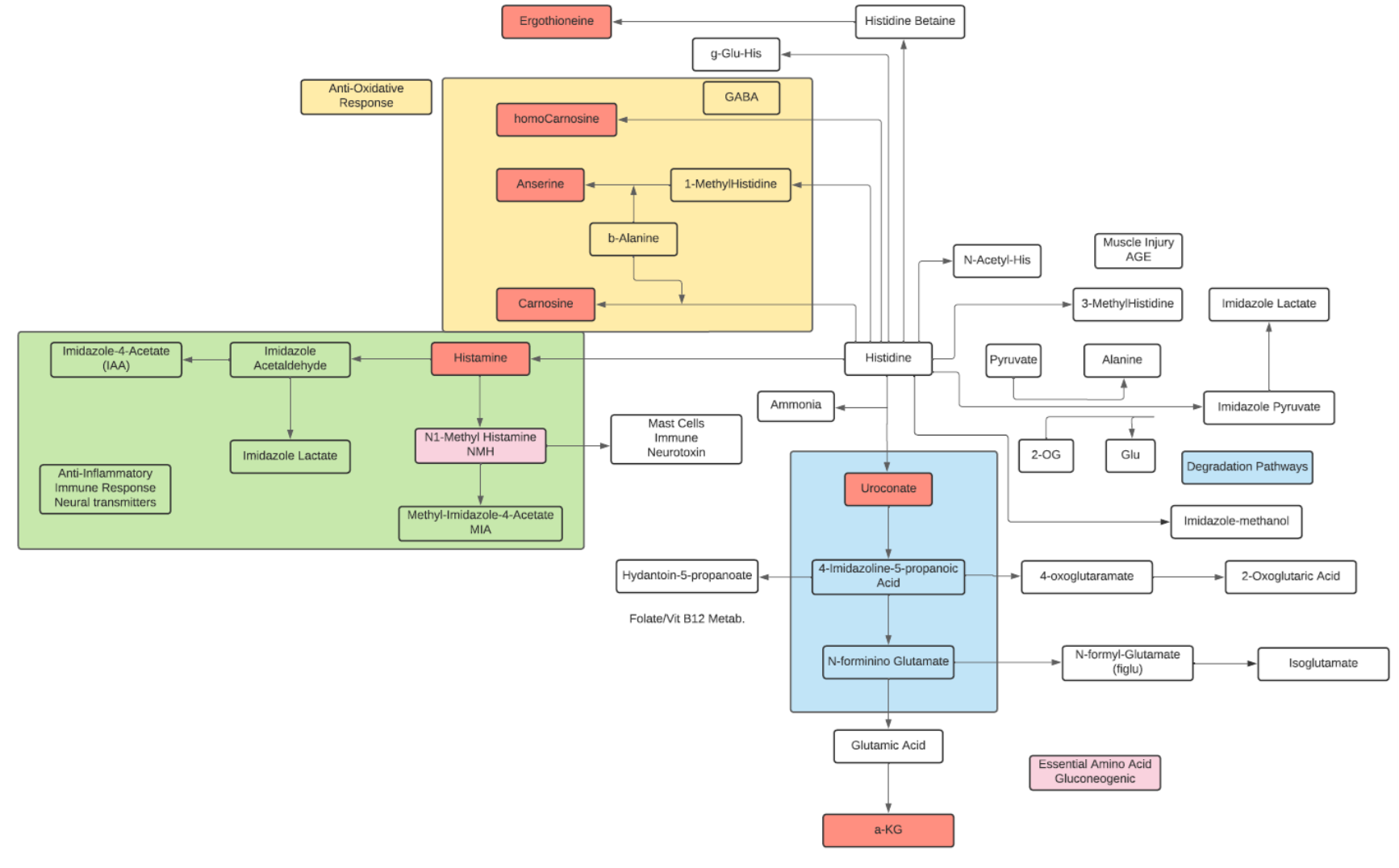
Histidine
- Histidine is classified as an aliphatic, positively charged, and basic amino acid.
- Histidine forms complexes with many divalent metal ions as a chelator within small peptides and proteins.
- Histidine is an essential amino acid in humans and other mammals.
- Histidine is a precursor for histamine and carnosine biosynthesis.
- Histamine regulates functions in the gut and acts as a neurotransmitter for the brain and spinal cord. Histamine is produced by basophils and by mast cells and increases the permeability of capillaries to white blood cells.
- Carnosine is a dipeptide of beta-alanine and histidine that is highly concentrated in muscle and brain. Carnosine is naturally produced in the liver. Carnosine scavenges reactive oxygen species (ROS) as well as alpha-beta unsaturated aldehydes formed from peroxidation of fatty acids during oxidative stress. It also acts as a neurotransmitter in the brain.
- Histidine and other imidazole compounds have antioxidant properties. The efficacy of L-histidine in protecting inflamed tissue is attributed to the capacity of the imidazole ring to scavenge reactive oxygen species (ROS) generated by cells during acute inflammatory response.
- can inhibit cytokines and growth factors involved in cell and tissue damage. Histidine appears to suppress pro-inflammatory cytokine expression, possibly via the NF-kappa-B pathway in adipocytes.
- The main pathway of catabolism begins with production of trans-urocanate and ammonia; in the skin considered a significant source of blood ammonia in the systemic circulation. The urocanate produced in the liver is hydrolyzed to formiminoglutamate (FIGLU), and FIGLU is converted to glutamic acid.
- The major histidine derivatives are 3-methylhistidine, whose plasma concentration and urine excretion are markers of myofibrillar protein degradation, 1-methylhistidine, produced from anserine in muscle, and ergothioneine, produced by cyanobacteria, mycobacteria, and fungi.
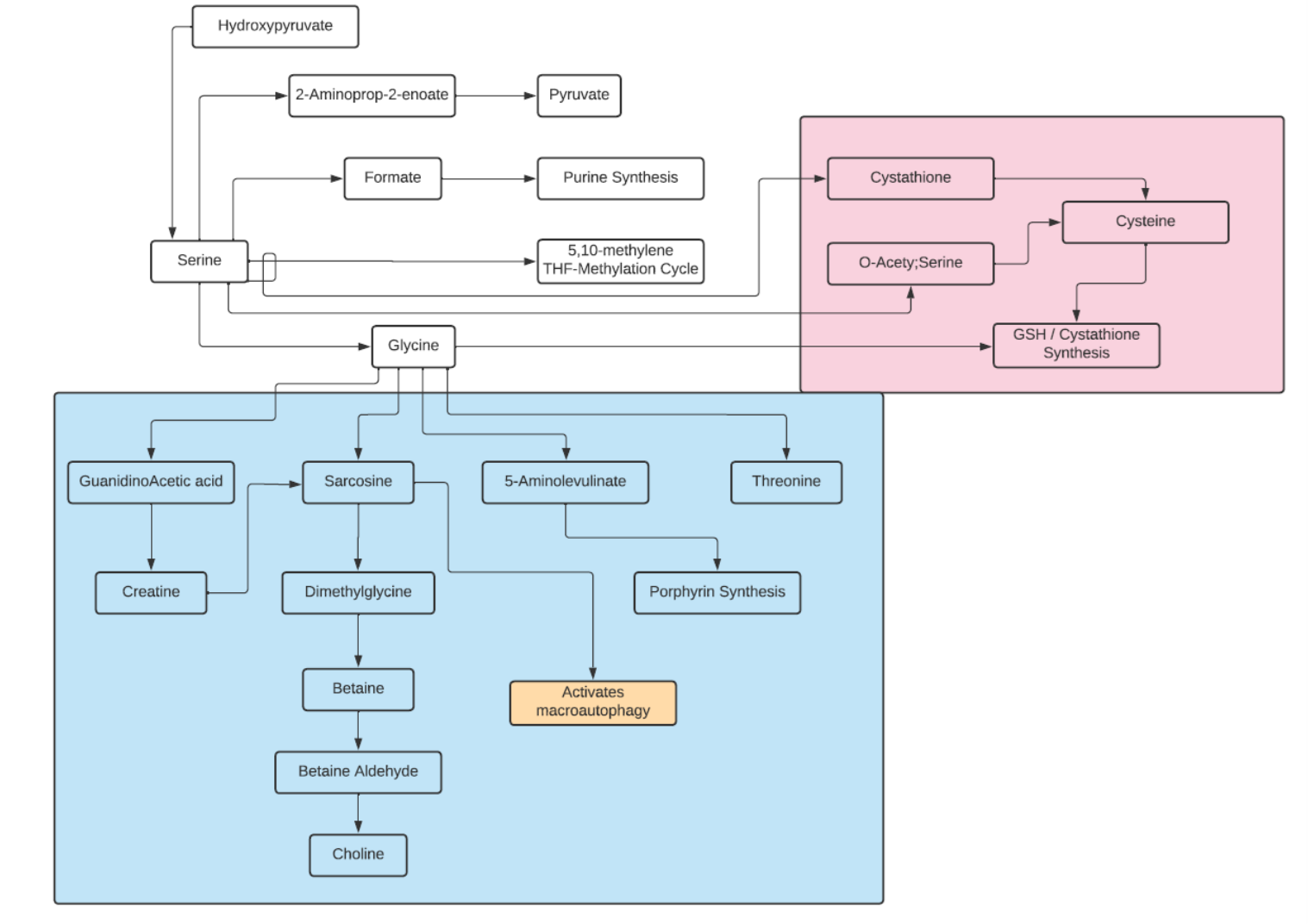
Glycine
- It is classified as an aliphatic, non-polar amino acid.
- In humans, glycine is a nonessential amino acid
- It is the only achiral proteinogenic amino acid.
- The name comes from the Greek word glucus or “sweet tasting”.
- Glycine is biosynthesized in the body from the amino acid serine, which is in turn derived from 3-phosphoglycerate.
- In addition to being synthesized from serine, glycine can also be derived from threonine, choline or hydroxyproline via inter-organ metabolism of the liver and kidneys.
- The predominant degradation pathway catalyzes the oxidative conversion of glycine into carbon dioxide and ammonia, with the remaining one-carbon unit transferred to folate as methylenetetrahydrofolate. It is the main catabolic pathway for glycine and it also contributes to one-carbon metabolism.
- Most proteins incorporate only small quantities of glycine, a notable exception being collagen, which contains about 35% glycine.
- In higher eukaryotes, delta-aminolevulinic acid, the key precursor to porphyrins (needed for hemoglobin and cytochromes), is biosynthesized from glycine and succinyl-CoA by the enzyme ALA synthase.
- Glycine provides the central C2N subunit of all purines, which are key constituents of DNA and RNA.
- Glycine is an inhibitory neurotransmitter in the central nervous system, especially in the spinal cord, brainstem, and retina.
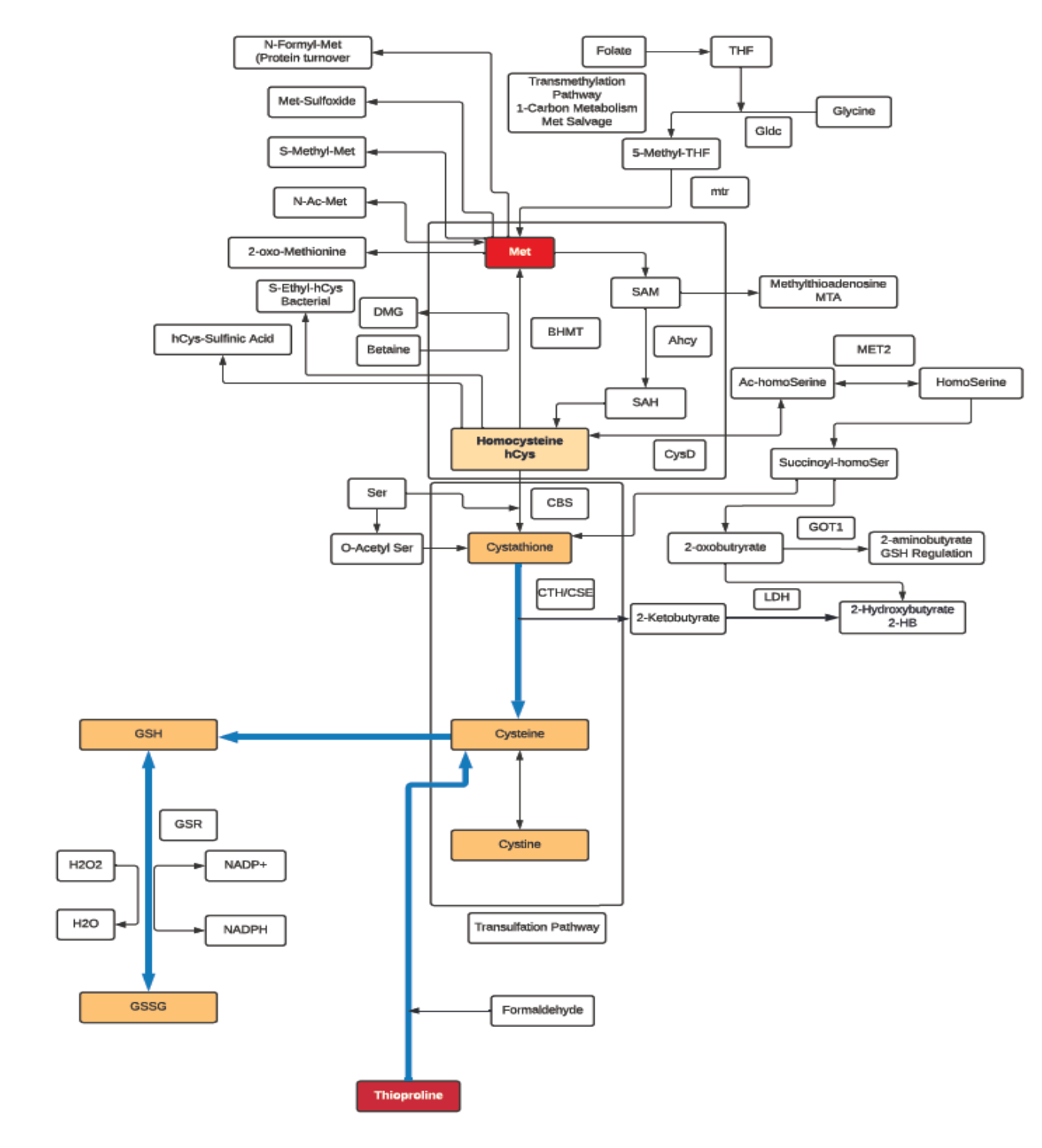
Methionine
- Methionine is classified as an aliphatic, non-polar amino acid.
- Methionine is a sulfur-containing, essential amino acid, and a precursor of succinyl-CoA, homocysteine, cysteine, creatine, taurine and carnitine.
- In addition to being a substrate for protein synthesis and an intermediate in transmethylation reactions, it serves as the major methyl group donor in vivo, including the methyl groups for DNA and RNA intermediates.
- It is a methyl acceptor for 5-methyltetrahydrofolate-homocysteine methyltransferase (Methionine synthase); the only reaction that allows for the recycling of this form of folate, and is also a methyl acceptor for the catabolism of betaine.
- Methionine is the metabolic precursor for Cysteine (CYS). Only the sulfur atom from Methionine is transferred to Cysteine; the carbon skeleton of Cysteine is donated by serine!
- Acute doses of Methionine can lead to increases in plasma Homocysteine (hCys), which can be used as an index of the susceptibility to cardiovascular disease.
- Can regulate metabolic processes, the innate immune system, and digestive functioning in mammals.
- Intervenes in lipid metabolism, activates endogenous antioxidant enzymes such as methionine sulfoxide reductase A, and the biosynthesis of glutathione to counteract oxidative stress.
- Methionine restriction prevents altered methionine/transmethylation metabolism, thereby decreasing DNA damage and carcinogenic processes and possibly preventing arterial, neuropsychiatric, and neurodegenerative diseases.
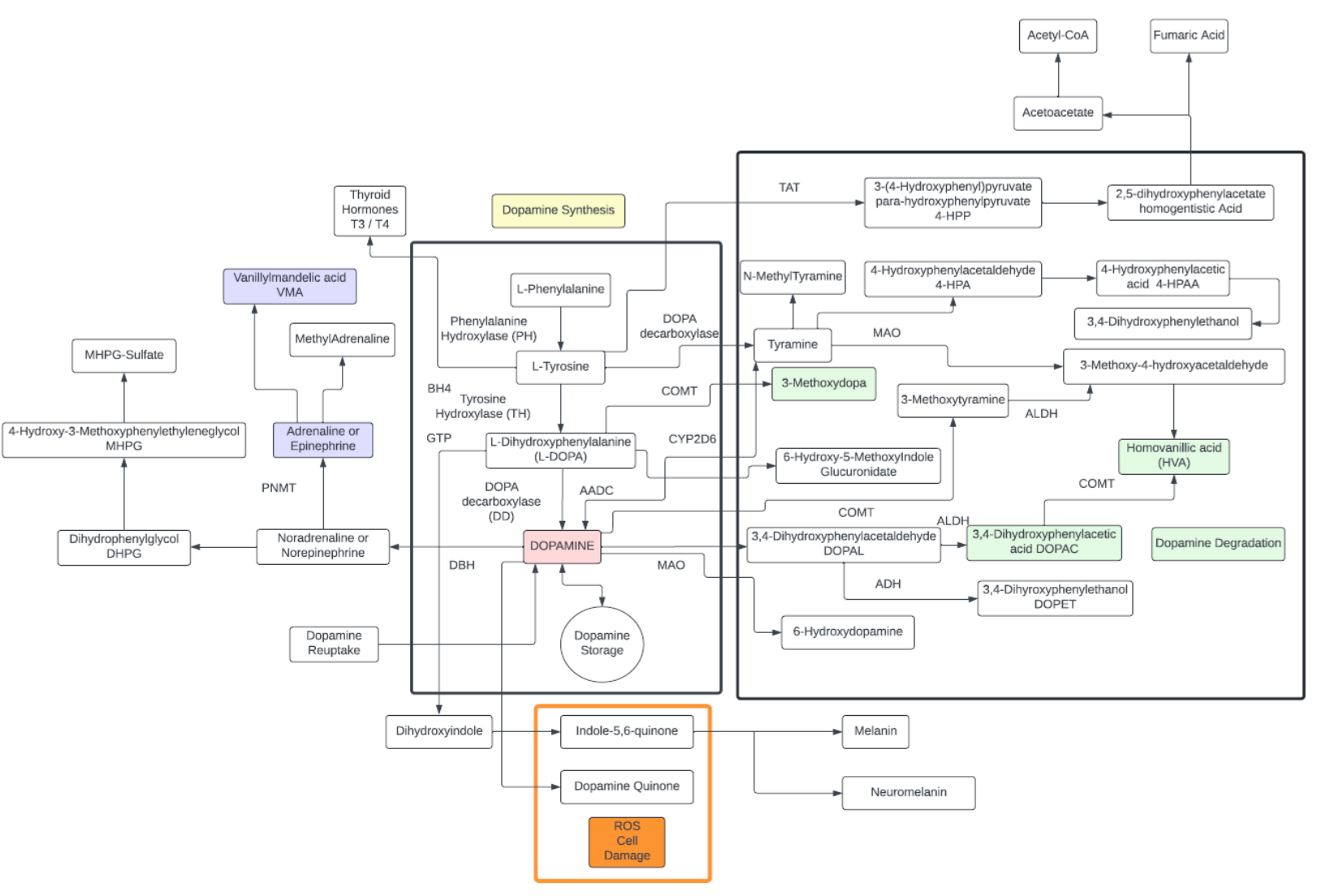
Tyrosine
- A non-essential amino acid with a polar side group, naturally produced largely in the liver from phenylalanine.
- One of the few amino acids that readily passes through the blood-brain barrier.
- Starting precursor to a number of well-known neurotransmitters including: Dopamine, L-DOPA, adrenaline, noradrenaline, additionally, thyroid hormones (T3,T4) and the pigment melanin:
- Three structural isomers of L-tyrosine are known. In addition to the common amino acid L-tyrosine, which is the para isomer, there are two additional regioisomers, namely meta-tyrosine (also known as 3-hydroxyphenylalanine, L-m-tyrosine, and m-tyr) and ortho-tyrosine (o-tyr or 2-hydroxyphenylalanine), that occur in nature. The m-tyr and o-tyr isomers, which are rare, arise through non-enzymatic free-radical hydroxylation of phenylalanine under conditions of oxidative stress.
- Plasma tyrosine as a precursor of noradrenaline in the brain might be a valuable biomarker to predict brain glycogen dynamics.
- Tyrosine is mainly degraded in the liver where alterations in degradation pathways can be indicative of liver disease.
- The degraded product, homogenistic acid, can be further broken down to Acetyl-CoA and fumaric acid to feed into the TCA cycle.
- Tyrosine is not found in large concentrations throughout the body, probably because it is rapidly metabolized.
- Tyrosine is needed to synthesize the benzoquinone structure which forms part of coenzyme Q10.
- Altered Tyrosine in serum has been associated with insulin resistance as an inflammatory marker and indicative of impaired glucose metabolism prior to diagnosis of metabolic syndrome.
- Blood tyrosine levels can be a biomarker of non-alcoholic fatty liver disease.
- High tyrosine concentrations have also been detected in septic patients.
Citrulline
- Citrulline was first isolated from watermelon in 1914 by Japanese researchers Yotaro Koga and Ryo Odake and further codified by Mitsunori Wada of Tokyo Imperial University in 1930.
- Citrulline is a neutral, non-essential amino acid and a major precursor of Arginine in the nitric oxide (NO) cycle.
- Citrulline can be biosynthesized from carbamoyl phosphate and Ornithine.
- Associated with several diseases such as ulcerative colitis, Parkinson’s, dementia, ED, high blood pressure, diabetes and rheumatoid arthritis.
- Proteins that normally contain citrulline residues include myelin basic protein (MBP), filaggrin, and several histone proteins.
- Citrulline is also produced as a byproduct of the enzymatic production of nitric oxide from the amino acid arginine, catalyzed by a nitric oxide synthase (iNOS, eNOS, or nNOS).
- Mainly synthesized in the liver and intestinal membranes and can be a biomarker for gut health.
- CItrulline prevents neuronal cell death and protects cerebrovascular injury, therefore, Citrulline may have a neuroprotective effect to improve cerebrovascular dysfunction
- Citrulline supplements have been claimed to promote energy levels, stimulate the immune system and help detoxify ammonia.
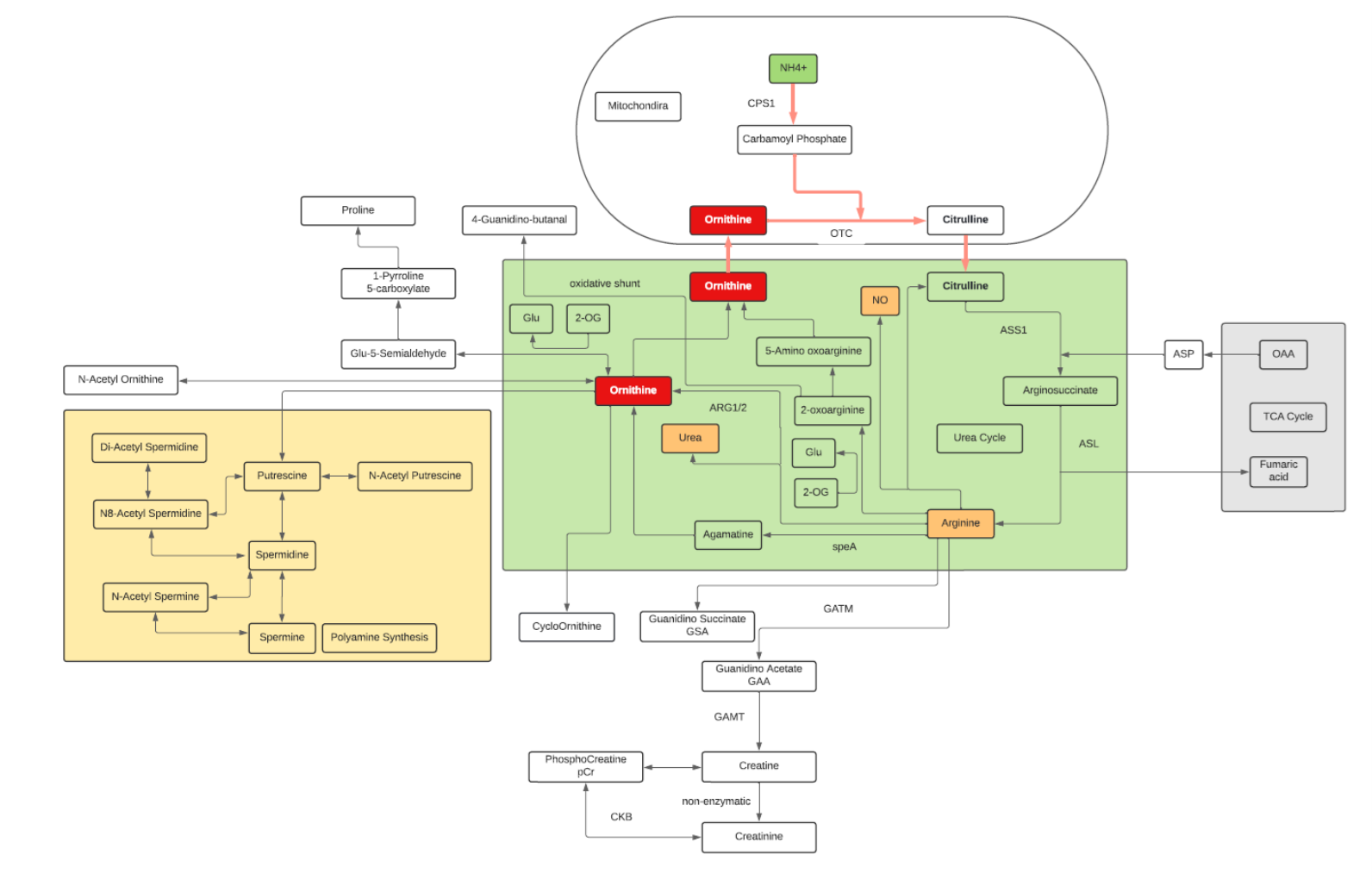
Ornithine
- Ornithine is one of the key reactants in the urea cycle, responsible for 80% of the nitrogen excretion in the body.
- Ornithine enhances liver function and helps detoxify harmful substances.
- L-Ornithine is one of the products of the action of the enzyme Arginase on L-Arginine, creating urea. In mammalian non-hepatic tissues, the main use of the urea cycle is in Arginine and Citrulline biosynthesis.
- Ornithine is converted into citrulline by action of ornithine transcarbamylase. Ornithine binds with carbamoyl phosphate which requires ammonia to be produced and then is converted into Citrulline giving off urea as a byproduct. Due to this, the conversion is one that reduces ammonia concentrations in the blood and concomitantly increases urea.
- In the liver, human ORC1 catalyzes the citrulline/ornithine exchange across the mitochondrial inner membrane, which is required for the urea cycle. Human ORC1, ORC2, and SLC25A29 are likely to be involved in the biosynthesis and transport of Arginine, which can be used as a precursor for the synthesis of NO, Agmatine, polyamines, Creatine, Glutamine, Glutamate, and Proline, as well as in the degradation of basic amino acids.
- Associated with Hepatic encephalopathy, Ornithine is dysregulated in people with chronic liver disease, such as cirrhosis or hepatitis.
- ORN may have immunomodulatory and wound-healing activities in disease (by virtue of its metabolism to L-arginine).
- Ornithine decarboxylase (ODC) initiates the polyamine biosynthetic pathway. The amount of ODC is altered in response to many growth factors, oncogenes, and tumor promoters and to changes in polyamine levels. Ornithine decarboxylase (ODC) catalyzes the first step in the polyamine biosynthetic pathway forming putrescine, which is then converted into the polyamines spermidine and spermine. Polyamine content plays important roles in both normal and neoplastic growth and alterations of polyamine synthesis via changes in ODC content occur in response to tumor promoters and carcinogens.
- OAT is a nuclear-encoded pyridoxal phosphate (vitamin B6) requiring enzyme that catalyzes the conversion of excess ornithine – generated from arginine in the urea cycle – into pyrroline-5-carboxylic acid (P5C). As ornithine is a precursor of proline, a deficiency in OAT leads to decreased formation of Proline.
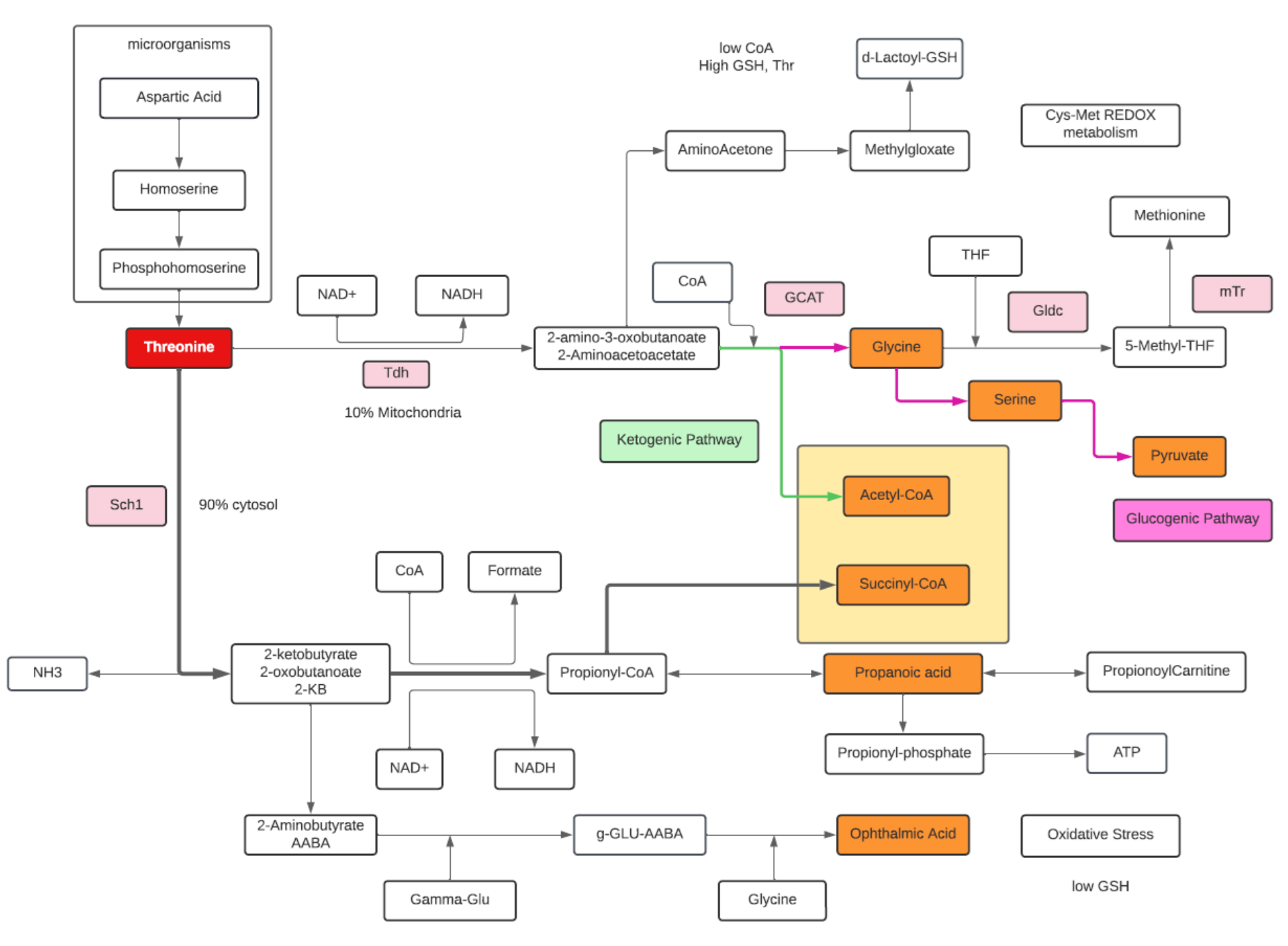
Threonine
- Threonine is an essential amino acid.
- Threonine is an amino acid that is both glucogenic (converted to pyruvate) and ketogenic (converted to Acetyl-CoA).
- A common pathway of Threonine degradation involves the formation of Acetyl-CoA and Glycine in the mitochondria in a two-step sequence. L-threonine dehydrogenase (Tdh) oxidizes Thr to 2-amino 3-ketobutyrate which in turn combines with CoA to form Glycine and Acetyl-CoA.
- Glycine is subsequently converted into Serine by serine hydroxymethyl transferase (ShT) , and then Serine in transformed into Pyruvate by serine dehydratase (SDh).
- However, the main flux of Thr catabolism occurs in the cytosol with generation of Propionyl-CoA from α-ketobutyrate.
- α-ketobutyrate can be converted to succinyl-CoA for oxidation in the citric acid cycle or serve as a precursor to Ophthalmic acid. Ophthalmate can be biologically synthesized from α-ketobutyrate through consecutive reactions with gamma-glutamylcysteine synthetase and glutathione synthetase. Ophthalmic acid can be used as a biomarker for oxidative stress when the depletion of glutathione occurs.
- Acetyl-CoA is required for carbon recyclization, whereas Glycine and its subsequent degradation are required for nitrogen recyclization.
- The deaminase/dehydratase or Propionyl-CoA pathway is an anaerobic pathway that produces Propionyl-phosphate, which contributes to ATP synthesis.
- Threonine is a limiting amino acid (the others are Lysine, Methionine, and Tryptophan). Limiting amino acids are found in the shortest supply from digested proteins.
- An alternative pathway from 2-amino-3-oxobutanoate is the Glyoxalase pathway which increases with high Glutathione concentrations, while the preference to α-ketobutyrate to Ophthalmic acid increases with low glutathione concentration.
- Most nitrogen from metabolized Thr is excreted into urine as urea. Aminoacetone and d-lactate are minor metabolic products whose urinary excretion increases disproportionately with high Thr intake and when availability of free CoA is limited.
- Threonine is an immunostimulant which promotes the growth of thymus gland. It also can probably promote cell immune defense function.
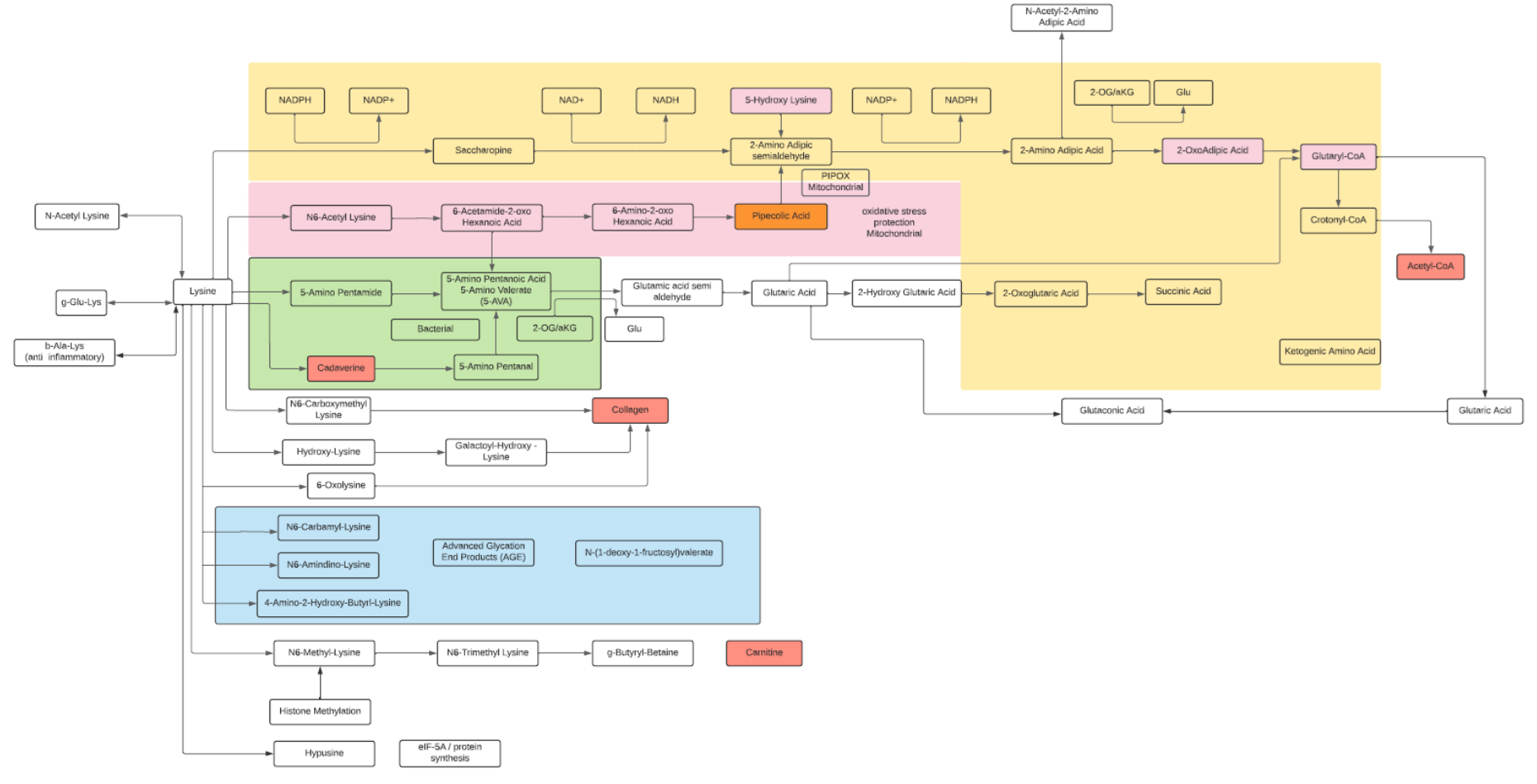
Lysine
- Lysine is an essential basic amino acid.
- Lysine is often used as a supplement for muscle growth.
- Methylated forms of lysine lead to carnitine synthesis (minor pathway, as most of carnitine is from diet).
- Common modifications are acetylation, methylation, hydroxylation (Collagen) and O-glycosylation. Other modified forms may reflect protein aging (advanced glycation end products or AGES).
- Modification of histones includes Lysine modifications as a major regulatory factor.
- The major degradation pathway is the Saccharopine pathway within liver mitochondria leading to Glutaryl-CoA and finally to Acetyl-CoA. Lysine and Leucine are the only two solely ketogenic amino acids.
- A secondary degradation pathway, the Pipecolate pathway, converges with the Saccharopine pathway. This pathway primarily occurs within the peroxisome, the site of β-oxidation of fatty acids, biosynthesis of phospholipids and metabolism of reactive oxygen species.
- Metabolites of Lysine, alpha amino adipic acid (AAA) from the Saccharopine pathway and pipecolic acid (PA) from the Pipecolate pathway are potential biomarkers. AAA is a potential predictor of the development of diabetes and modulator of glucose homeostasis. PA has been described as a biomarker for peroxisome dysfunction and certain cancers.
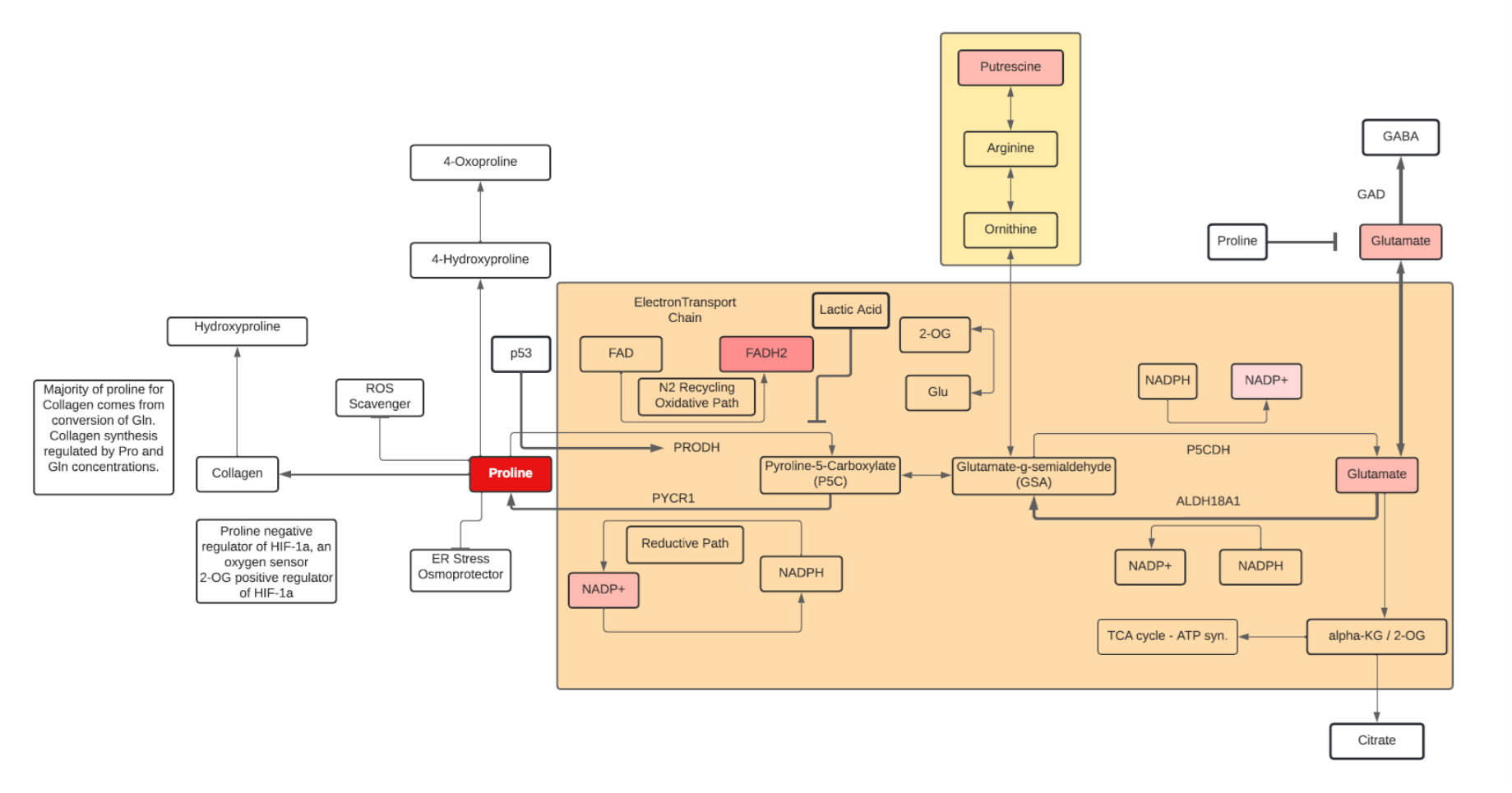
Proline
- Proline is a nonessential, sweet tasting amino acid. It contains a secondary α-imino group and is sometimes called an α-imino acid.
- Proline, and its secondary metabolite hydroxyproline, constitute a third of the total amino acids found in collagen. Lysine, proline, hydroxyproline, and vitamin C are all important in the synthesis of collagen for skin, bones, tendons, and cartilage. Proline is also commonly found in antimicrobial peptides, salivary proteins and cornified epidermis.
- In addition to dietary sources, proline can be synthesized from glutamate/glutamine, arginine, and ornithine. It can also be synthesized within enterocytes from degradation of small peptides.
- Proline has many physiologic functions, including:
- regulation of gene expression via mTOR activation (integrating nutrient and growth factor signaling in cells)
- cellular redox reactions
- amino acid (hydroxyproline and arginine) generation, and protein synthesis
- ROS scavenging (antioxidant activity)
- Small proline peptides can be bioactive: Pro-Pro induces NGF, Gly-Pro-Glu, Gly-Pro are anti inflammatory, while Pro-Gly-Pro has been found to be neuroprotective.
- Proline can be an energy source for bacteria and cancer cells by conversion to 2-OG and entering the TCA cycle.
- In addition to its role as ROS scavenging, Proline can act as an osmolyte and protein chaperone, further abating cellular stress
- Proline can participate in cell signaling by affecting levels of Glu and Arg (mTOR pathway) and promote Histone methylation and levels of 5-methylcytosine and 5-Hydroxymethylcytosine.
- Proline can stimulate growth of stem and tumor cells.
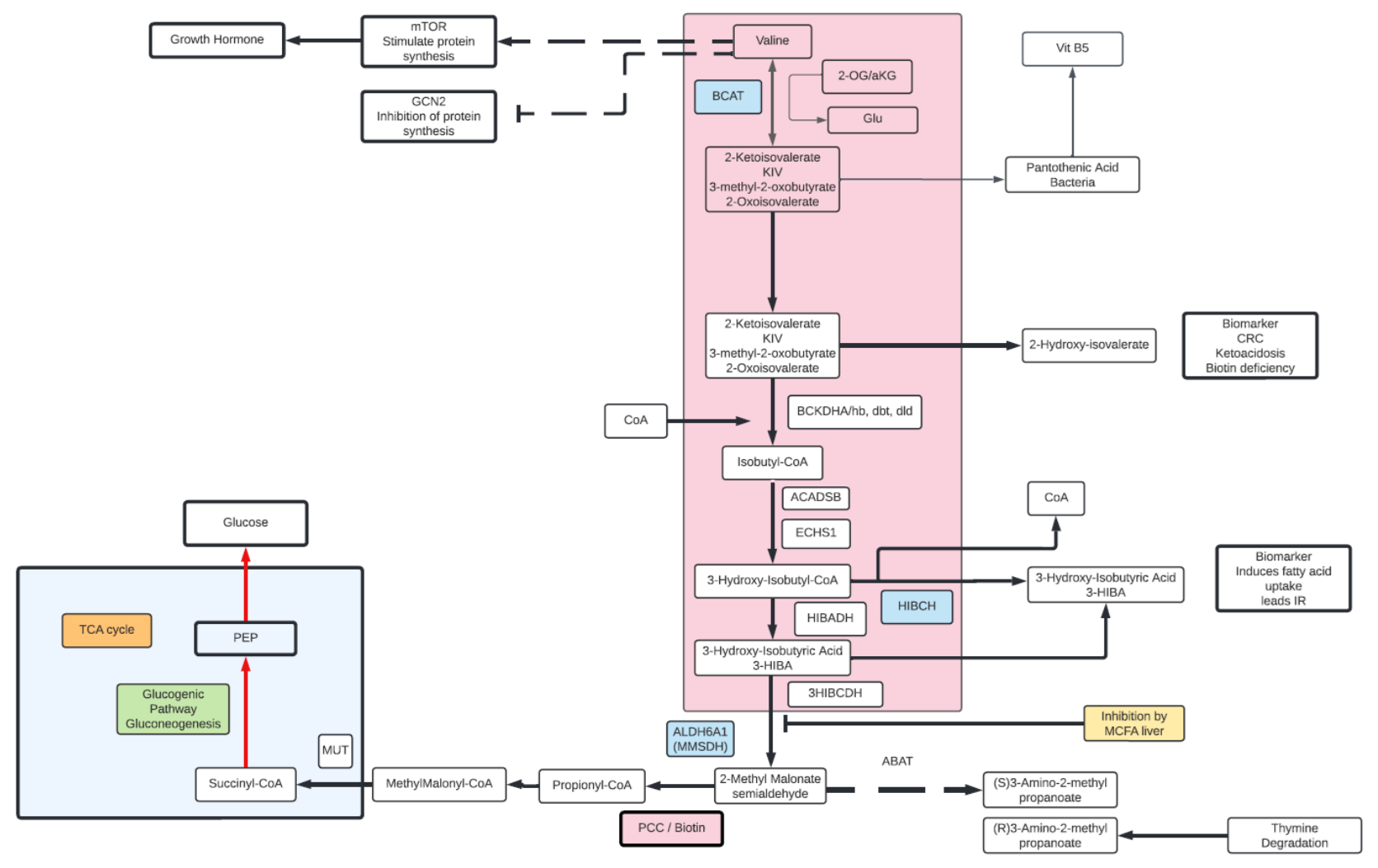
Valine
- Valine is an essential amino acid and a branched chain amino acid (BCAA).
- While an essential amino acid in humans, in plants and bacteria can be synthesized from pyruvate.
- Degradation of Valine starts with removal of the primary amino group via a transamination reaction with α-KG, yielding glutamate and α-ketoisovalarate.
- Ultimately, Valine is biotransformed into succinyl-CoA and enters the TCA cycle, hence Valine is a glucogenic amino acid and part of the gluconeogenesis pathway.
- β-aminoisobutyrate (2-AIB) is another Valine metabolite released by active muscle or in kidney as well as liver. High levels of 2-AIB may be indicative of VitB6 deficiency. 2-AIB can be metabolized into propionyl-CoA in a glucogenic pathway.
- High plasma Valine observed in Type 2 diabetic patients along with the Valine metabolite, 3-hydoxyisobutyrate (3-HIBA). 3-HIBA is linked to insulin sensitivity, increased uptake of fatty acids, lowering of plasma lipids and decreased blood glucose. 3-HIBA promotes the accumulation of fat within muscle tissue by stimulating fatty acid uptake into the muscle. accumulation of intramuscular fat activates certain signaling cascades within the muscle cell that diminish insulin signaling, leading to insulin resistance.
- Another end product of Valine degradation is (S) 3-amino-2-methyl propionic acid, can be observed under starvation or stress. A homolog (R) 3 amino-2-methyl proprionic acid is formed from the degradation of Thymine and unrelated to Valine metabolism.
- Along with other BCAAs (isoleucine, Leucine) Valine is associated with enhanced energy, increased endurance, and aids in muscle tissue recovery and repair presumably through glucogenic catabolism leading to enhancing TCA cycle intermediates and gluconeogenesis.
- Valine has been linked to protein synthesis through its interactions with the mTOR and GCN2 pathways, leading to activation of growth hormones as well.
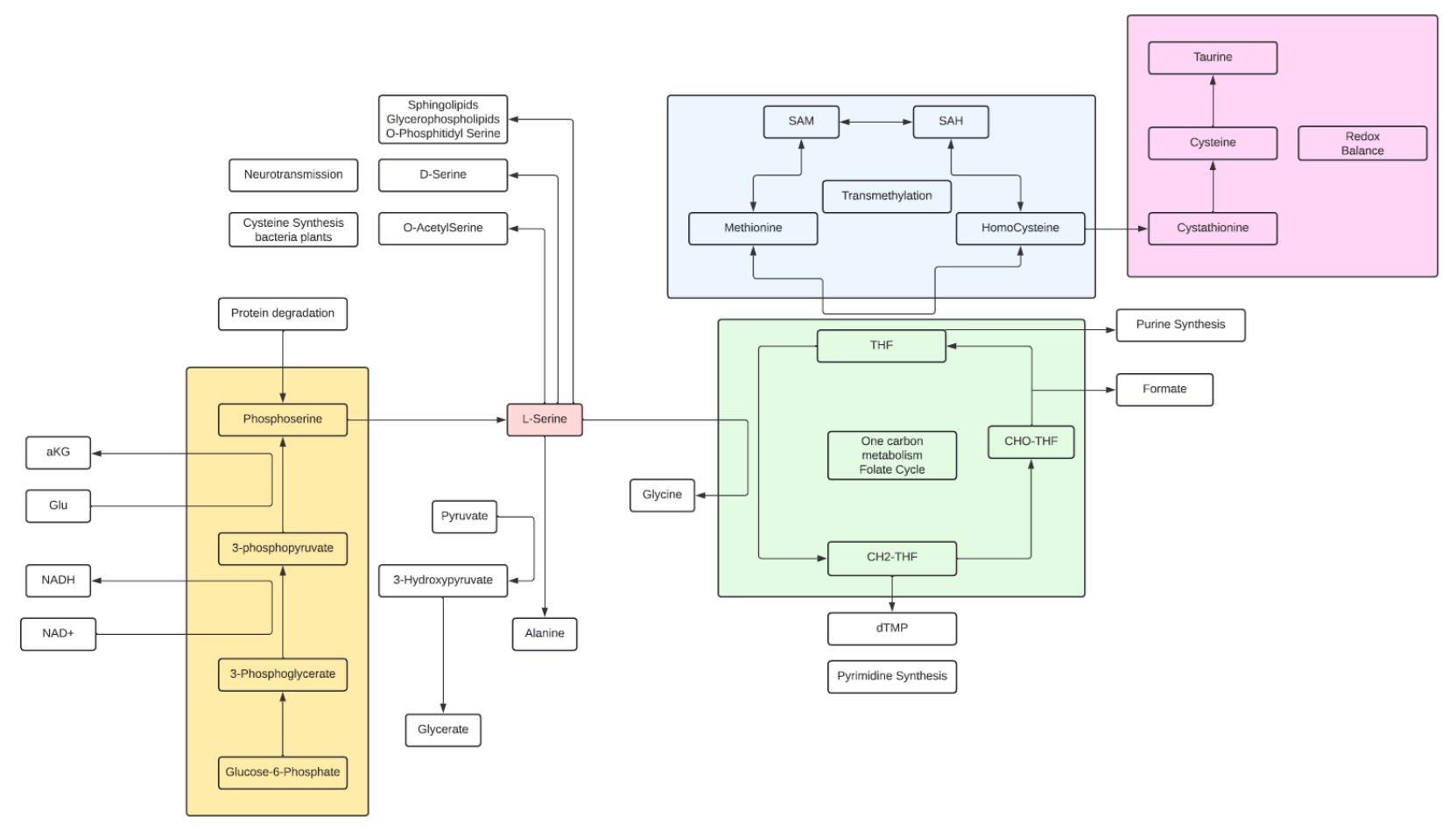
Serine
- Serine is a nonessential amino acid and a polar metabolite
- Serine is a major contributor to the one-carbon pool and in the synthesis of glycine and cysteine and Taurine, as well as, phospholipids such as phosphatidylserine, which is a component of the membrane of brain cells.
- L-Serine is one of two amino acids (the other is Aspartate) where the D isomer is fairly common with known biological significance. D-Serine has a critical role as a neuromodulator in the brain.
- D-Serine can be synthesized in neurons by serine racemase from L-serine, serving as a neuromodulator by coactivating NMDA receptors, making them able to open if they then also bind glutamate. D-serine is a potent agonist at the glycine site of the NMDA-type glutamate receptor. For the receptor to open, glutamate and either glycine or D-serine must bind to it. In fact, D-serine is a more potent agonist at the glycine site on the NMDAR than glycine itself.
- As one of two amino acids that are cited with biological active D isomers, D-Serine and D-aspartate are suggested to be the only D-amino acids in the human body originating from tissue intrinsic racemization. Therefore, these D-amino acids are referred to as canonical D-amino acids, and they are the two most commonly studied D-amino acids in relation to human physiology and cancer.
- The biosynthesis of L-Serine starts with the of 3-phosphoglycerate to 3-phosphohydroxypyruvate and NADH by phosphoglycerate dehydrogenase. Reductive amination of this ketone by phosphoserine transaminase yields 3-phosphoserine which is hydrolyzed to Serine by phosphoserine phosphatase.
- Serine hydroxymethyltransferase (SHMT) catalyzes the reversible conversions of L-Serine to Glycine and 5,6,7,8-tetrahydrofolate (THF) to 5,10-methylenetetrahydrofolate (mTHF).
- Typically, 1/3 of the Serine pool goes into protein synthesis and about 25% into Glycine synthesis, the remaining goes into other pathways.
Leucine
- Leucine is an essential amino acid and one of three branched chain amino acids (others are Isoleucine and Valine). The Leucine side chain is an isobutyl group, making it a non-polar aliphatic amino acid.
- The carbon skeleton of leucine can be used to generate ATP by degradation to Acetyl-CoA. Hence, Leucine is one of the two exclusively ketogenic amino acids, with lysine being the other. It is the most important ketogenic amino acid in humans.
- The degradation product of Leucine, β-hydroxy β-methylbutyric acid (HMB) has shown pharmacological activity in humans and has been demonstrated to promote protein biosynthesis via the phosphorylation of mTOR, a serine-threonine protein kinase that regulates protein biosynthesis and cell growth. High blood levels of
- Leucine are associated with insulin resistance. This might be due to Leucine stimulation of mTOR signaling.
Sestrin2 connects the concentration of Leucine to the control of organism metabolism and growth. When Leucine is bound to Sestrin2, it is released from a complex with the mTORC1 regulatory factor GATOR2, activating the mTORC1 complex. - Leucine can regulate several cellular processes such as protein synthesis, tissue regeneration, and metabolism. The level of Leucine tells an organism a lot about its physiological state, including how much food is available, how much insulin is going to be needed, and whether new muscle mass can be made.
- Most dietary Leucine is metabolized within the liver, but Leucine levels are higher in adipose tissue and muscle. Leucine is the highest BCAA amino acid in muscle tissue.
- Around 5% of Leucine ends up as HMB and another 40% to Acetyl-CoA. Acetyl-CoA can then lead to multiple metabolism – fatty acid synthesis or histone acetylation or energy ATP generation.
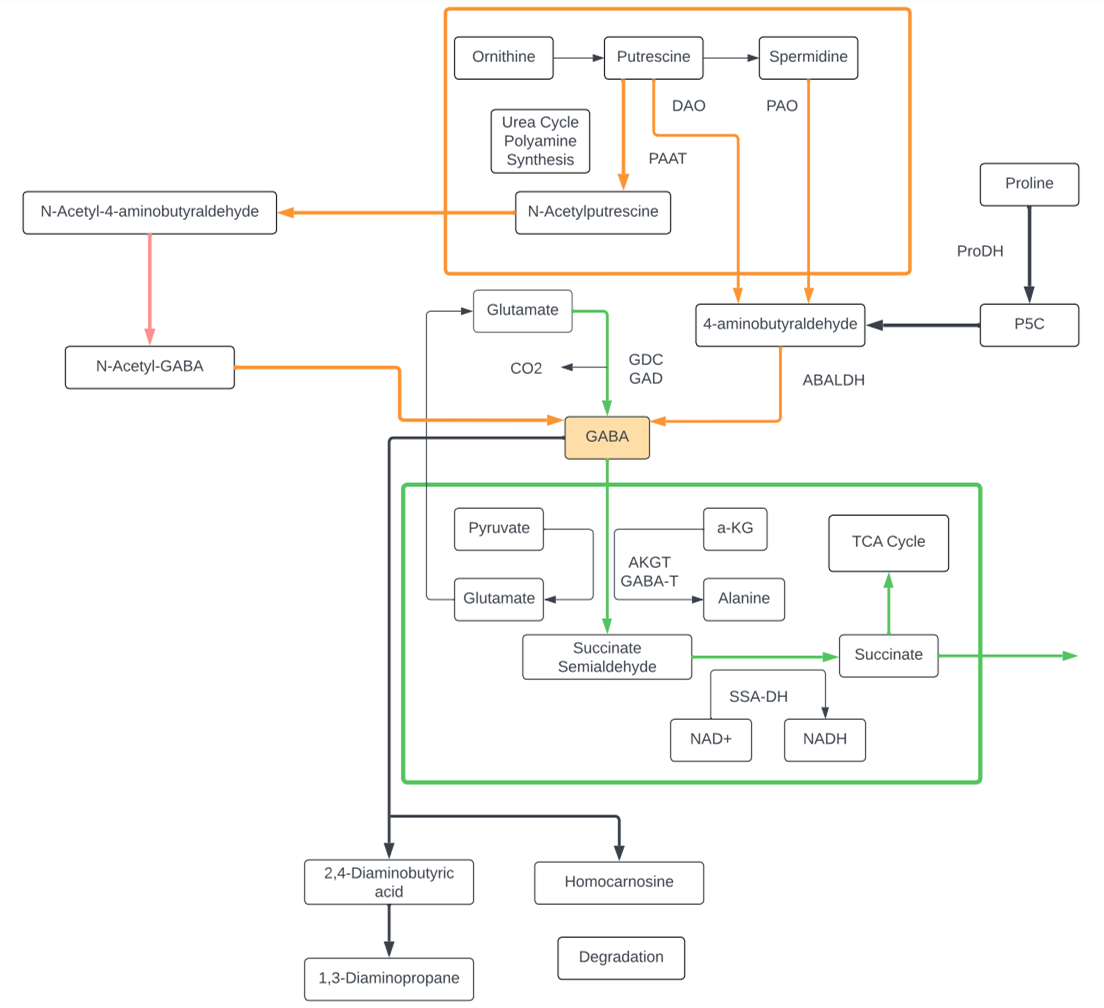
GABA
- GABA, or gamma-aminobutyric acid, is a non-proteogenic amino acid found ubiquitously in microbes, plants, and animals. While first discovered in microbes, GABA has been found to play an incredibly important role in neurotransmission of higher order animals.
- Glutamate and GABA, respectively, comprise the primary excitatory and inhibitory neurotransmitters in mammals: this ‘balance’ is crucial for normal development and function of the nervous system.
- In humans, GABA signal transmission is conducted through two different receptors, GABAA and GABAB. GABAA is a fast acting, ionotropic receptor, while GABAB is a G-protein coupled receptor, whose activity is much slower. This temporal differentiation of activation allows the same GABA signal to have fine tuned effects, depending on the local environment.
- While GABA is, indeed, crucial for humans and other mammals, the gamma amino acid is also crucial in plants and lower order eukaryotes. GABA levels have been shown to increase in plants in response to environmental stress, such as drought and insect attacks. Interestingly, C. elegans, a species of roundworm nematode, utilizes GABA as the primary transmitter at neuromuscular synapses; much of what we know about GABA and its receptors have come from studies using C. elegans.
- In the mammalian nervous system, GABA is primarily thought to be converted from the excitatory neurotransmitter and amino acid, Glutamate. This is thought to be preceded by Glutamate’s conversion from Glutamine, which can be taken up from the cellular external environment. Plants can synthesize GABA via a specialized shunt in the TCA cycle, and has also been shown to be produced via the polyamine pathways.
- Defects in human GABA signaling has been linked to schizophrenia, anxiety, and other neurocognitive disorders. Genetic studies have revealed that single nucleotide polymorphisms within the genes that code for ionotropic GABA receptor are sufficient to increase prevalence of these disorders.
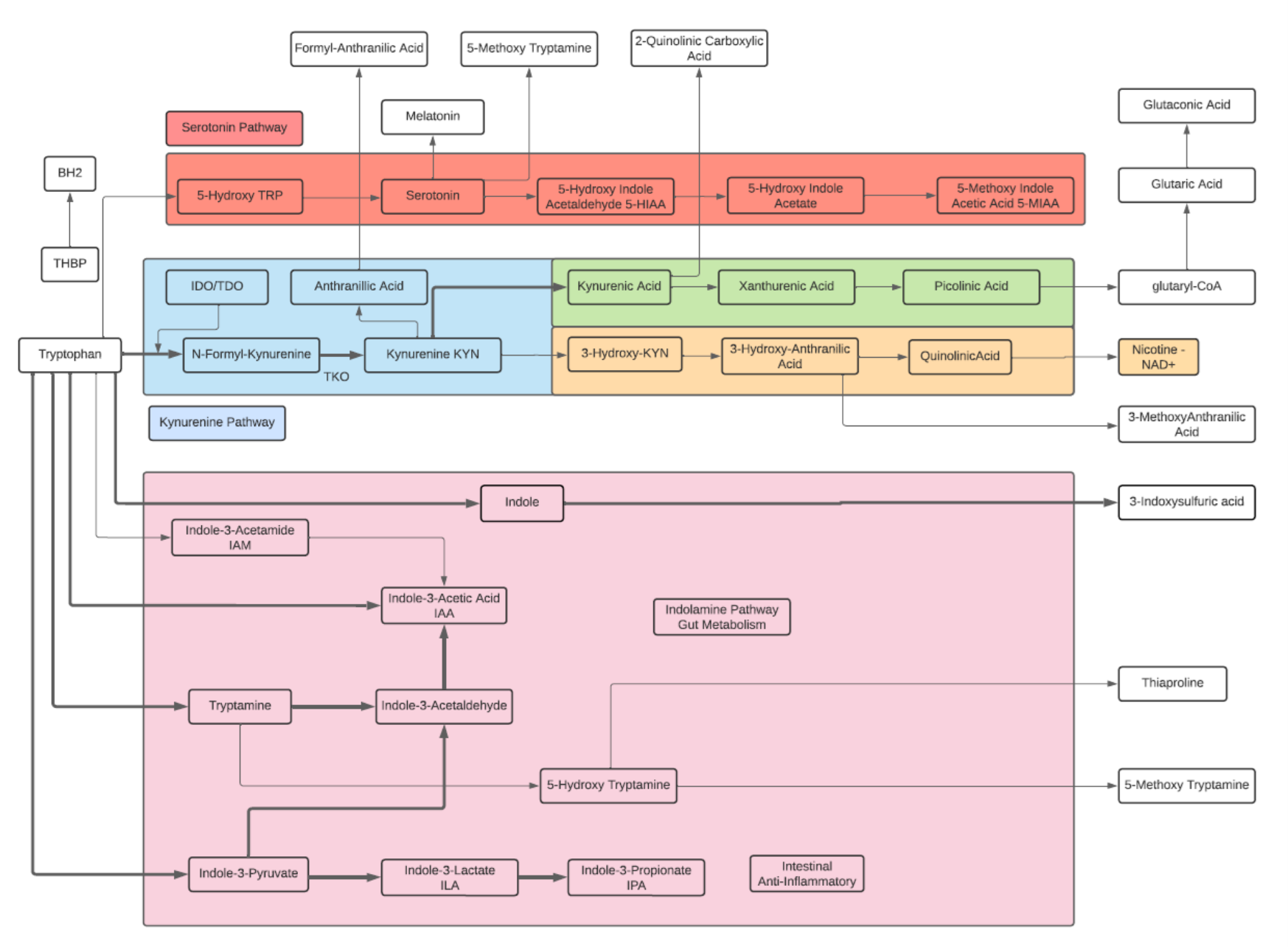
Serotonin
- Serotonin or 5-hydroxytryptamine (5-HT) is a monoamine neurotransmitter and a hormone. Its biological function is complex and multifaceted, modulating mood, cognition, reward, learning, memory, body temperature, sleep, sexual behavior and hunger.
- Lack of Serotonin may lead to depression, anxiety or mania.
- 90% of Serotonin is generated within the gastrointestinal tract from dietary Tryptophan where it may transfer into blood, and can be absorbed by platelets. Platelets then release Serotonin to help repair wounds by causing blood vessels to narrow, and slowing blood flow that helps clot formation. In the digestive tract, Serotonin helps to control bowel function, digestion, and appetite. Nausea can be triggered when Serotonin released from the gut faster than synthesized or replaced.
- About 10% of Serotonin is synthesized in brain tissue (known as the serotonergic system) within the brain stem. Along with Dopamine, Serotonin plays a role in sleep cycle.
- Serotonin is known as a “Happy Metabolite”, along with Dopamine, Oxytocin and Endorphins.
- Serotonin is a precursor to Melatonin and has been linked to growth hormone and DHEA levels. Lower levels of Serotonin is linked with aging, lower growth hormone and lower DHEA-Sulfate.
- 5-HT also plays a role in maintaining bone density and high levels can weaken bones or lead to osteoporosis.
- Sunlight and physical activity can lead to higher levels of Serotonin. Probiotics may also provide higher levels.
- Low levels of vitamin B6 and vitamin D have been linked to decreased levels of Serotonin.
- Serotonin Reuptake Inhibitors (SRIs) are commonly prescribed antidepressants. These drugs are used to reduce the symptoms of depression by increasing the available pool of Seratonin used for signaling
- Another group of serotonin-based medications for treating depression are known as Serotonin-Norepinephrine Reuptake Inhibitors (SNRIs). These drugs block the reuptake of Serotonin, but they also work on Norepinephrine, another neurotransmitter that affects mood.
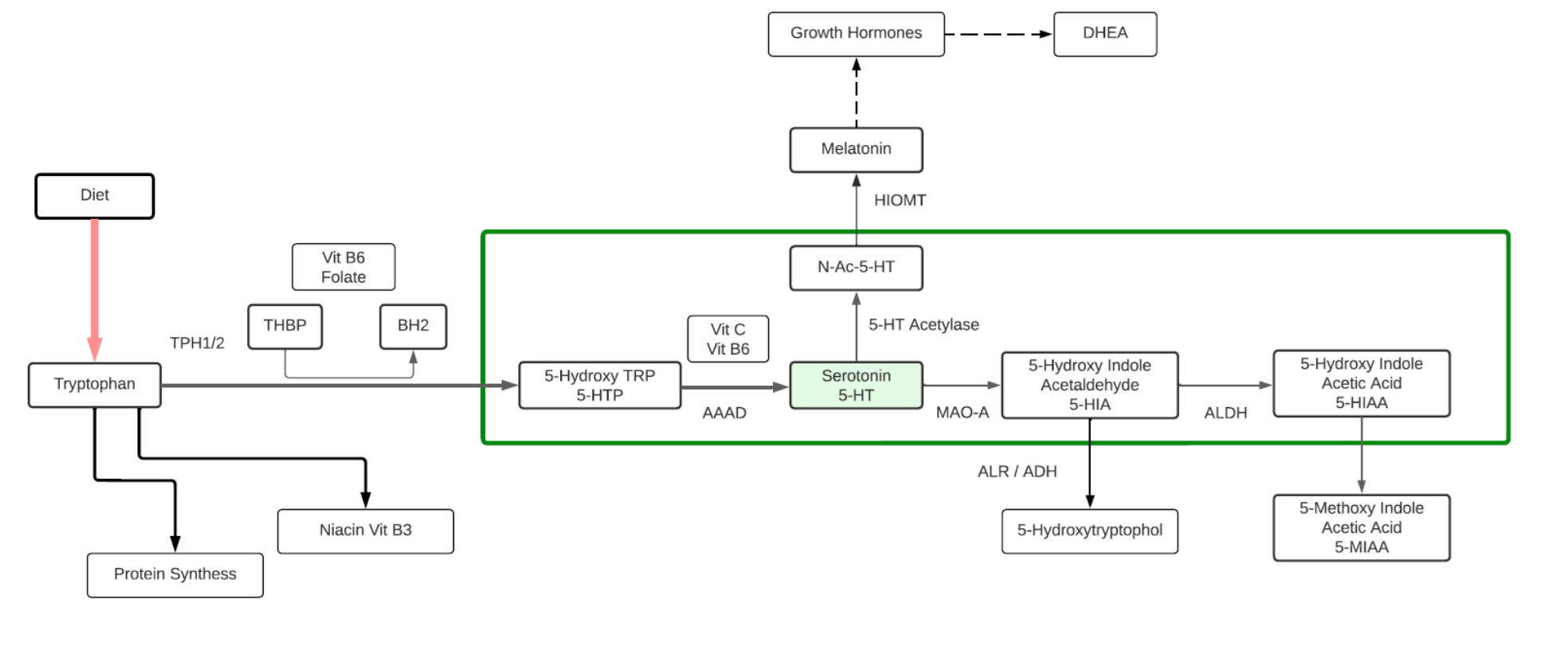
Tryptophan
- An essential aromatic amino acid.
- A precursor of Melatonin, a pineal gland hormone.
- A precursor of Vitamin B3 (Niacin).
- A precursor of Kynurenine, the major Trp endogenous metabolite, and links to the neuroprotective pathway (Picolinic acid), the neuro toxic pathway (Quinolinic Acid) and to NAD+.
- A precursor of phytohormones (Auxins) in plants.
- Some gut bacteria metabolize high levels of Trp metabolites including IPA, I3A, and Indoles that participate in the Gut-Brain neuroactive axis and Ahr receptors.
- The metabolite 5-Hydroxytryptophan (5-HTP) is decarboxylated to Serotonin (5-hydroxytryptamine or 5-HT) by the enzyme aromatic-L-amino-acid decarboxylase with the help of vitamin B6. This reaction occurs both in nervous tissue and in the liver. 5-HTP crosses the blood–brain barrier, while 5-HT does not.
- The metabolite Tryptamine is a ligand for gut epithelial serotonin type 4 (5-HT4) receptors and regulates gastrointestinal electrolyte balance through colonic secretions. Tryptamine can weakly activate the trace amine-associated receptor, TAAR1 (hTAAR1 in humans). Tryptamine may be a trace neuromodulator capable of regulating the activity of neuronal cell responses without binding to the associated postsynaptic receptor.
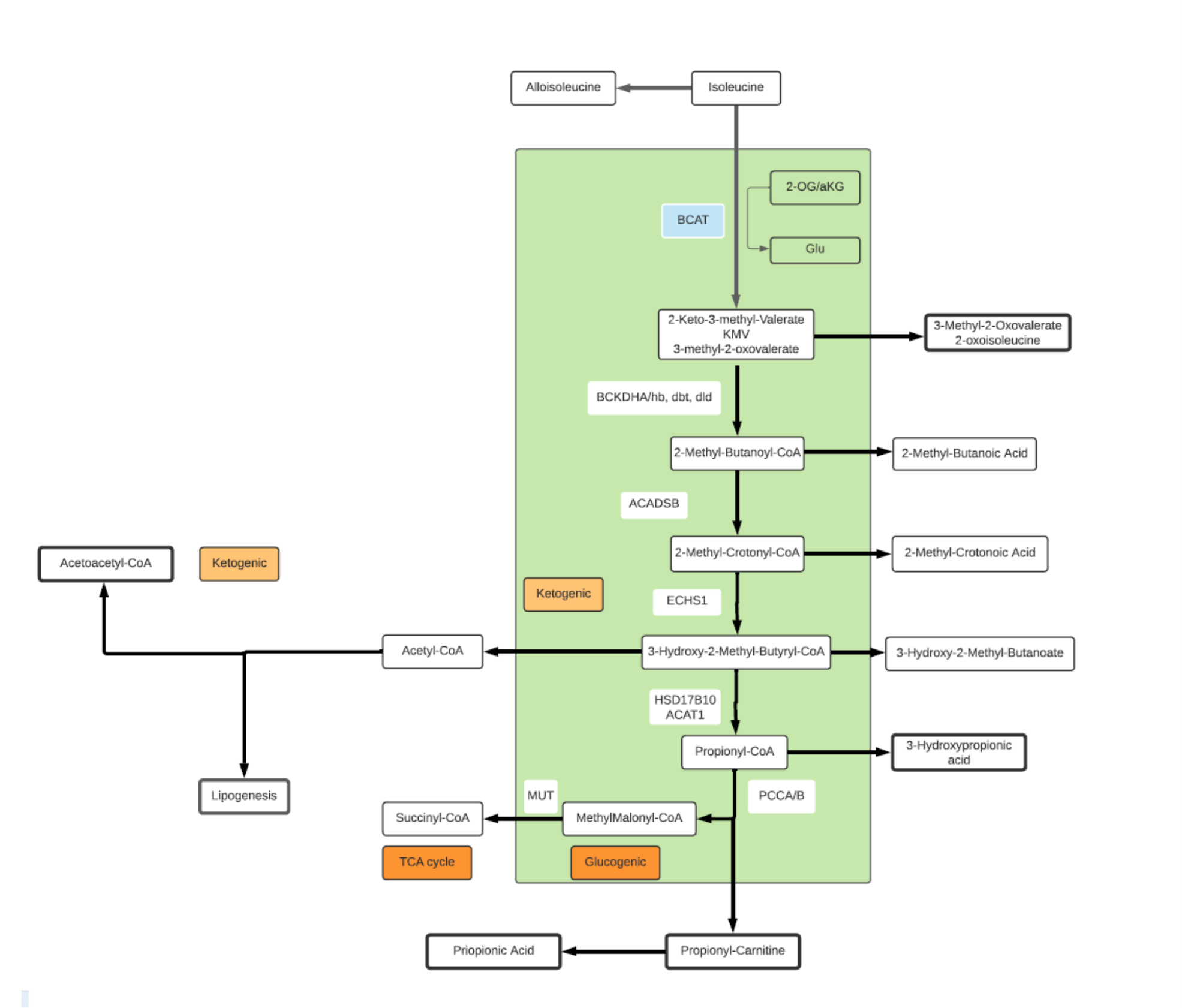
Isoleucine
- A branched, non-polar, aliphatic alpha amino acid.
- An essential amino acid in humans, synthesized by bacteria from pyruvate and 2-OG.
- Catabolism of Isoleucine plays an important role in health and disease.
- Both glucogenic (glucose building) and ketogenic (energy producing).
- End products of degradation include propionyl-CoA and acetyl-CoA.
- Propionyl-CoA is converted to Succinyl-CoA and enters the TCA cycle payoff phase contributing to both energy production and gluconeogenesis.
- Acetyl-CoA has multiple pathway participation including TCA cycle where it can transform into Citrate for fatty acid synthesis.
- BCAAs are associated with insulin resistance, high levels of Isoleucine are observed in diabetic and obese patients proportional to blood glucose levels.
- Isoleucine has 4 isomers: L-isoleucine, D-isoleucine, L-alloisoleucine and D-alloisoleucine.
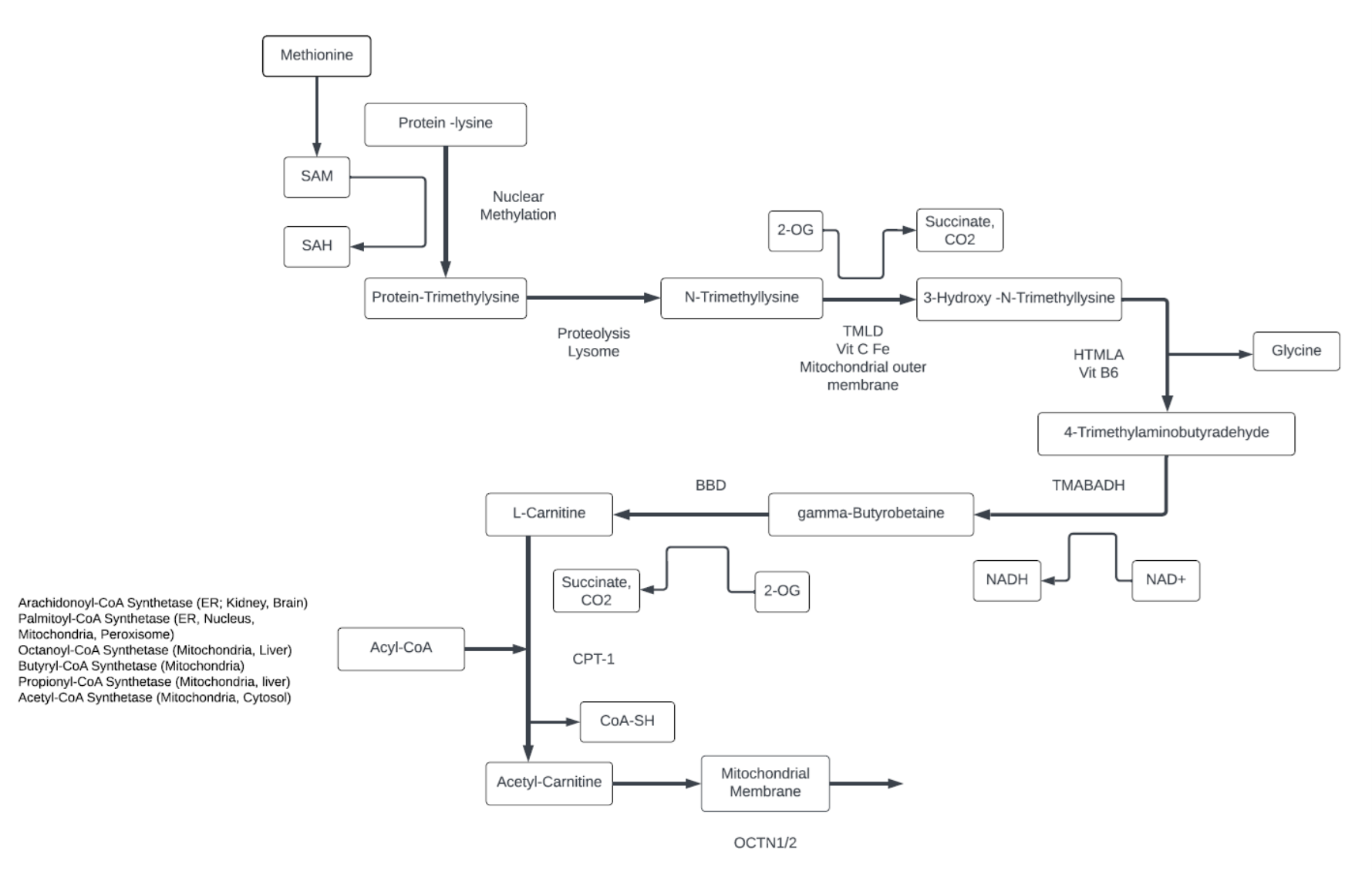
Carnitine
- Carnitine is derived from diet or synthesized from Lysine and Methionine. The carbon scaffold comes from the essential amino acid Lysine and methyl groups from Methionine.
- Most carnitine is stored in muscle tissue.
- Acyl-Carnitine is comprised of free carnitine and various forms of short, medium, and long-chain fatty acids (LCFAs) in its endogenous form as a fatty acid transporter. Carnitine can carry LCFAs through the mitochondrial membrane for beta-fatty acid oxidation, and remove short chain fatty acids from mitochondria.
- Long-chain fatty acids that are used as an energy source, are activated by the palmitoyl-CoA synthetase localized on the outer surface of the outer mitochondrial membrane , and converted by CPT-I at the same site.
- While middle chain fatty acids (MCFA) do not require carnitine transport in the liver, muscle and heart use MCFA-acyl carnitines. Carnitine stimulates mitochondria metabolism by decreasing Acyl-CoA and increasing CoA-SH.
- Carnitine has many metabolic functions, including:
- Stimulating hematopoiesis, restoring erythrocyte fluidity and enabling capillary transport.
- Inhibiting collagen-induced platelet aggregation.
- Preventing programmed cell death in immune cells and decreasing inflammatory cytokines.
- Modulating fatty acid oxidation.
- Preserving membrane integrity with membrane repair via reacylation.
- Stabilizing the coenzyme A (CoASH)/acetyl-CoA ratio in the mitochondria.
- Reducing lactate production.
- Carnitine may be involved in the pathogenesis of cancer development. There is evidence suggesting that carnitine-induces fatty acid oxidation, playing a critical role in the production of NADH, FADH2, NADPH and ATP, which could contribute to the development of tumors. Carnitine palmitoyltransferase I (CPTI) was reported to be overexpressed in numerous tumors, suggesting it may play an important role in tumor neovascularization. Higher plasma octanoylcarnitine levels are associated with lower breast cancer risk.
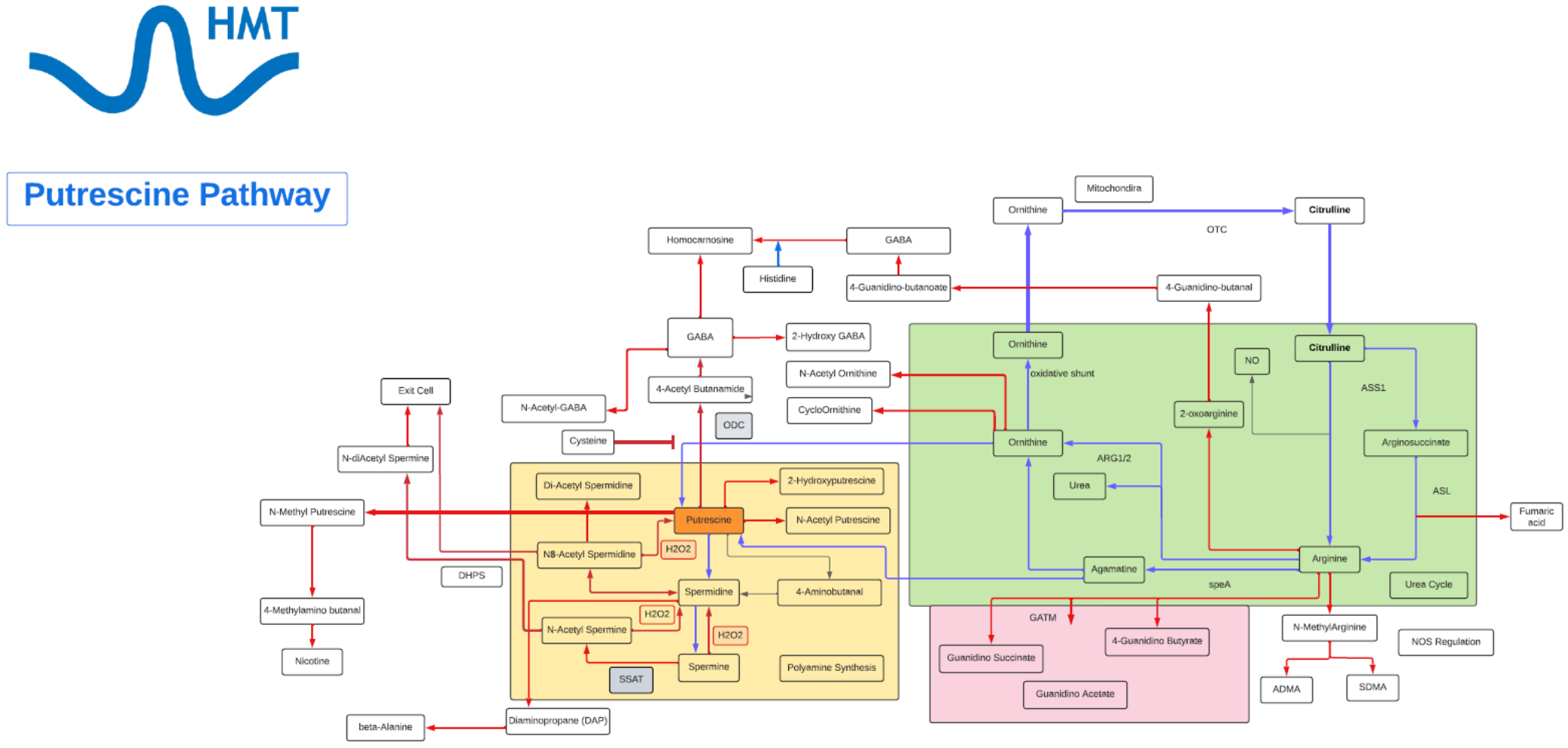
Putrescine
- Putrescine is a polyamine.
- Putrescine has been identified as a uremic toxin.
- Putrescine is synthesized in small quantities by healthy living cells via two different pathways, both starting from arginine.
- In one pathway, arginine is converted into agmatine. The conversion is catalyzed by the enzyme arginine decarboxylase (ADC). Agmatine is transformed into N-carbamoylputrescine by agmatine-imino hydroxylase (AIH). Finally, N-carbamoylputrescine is hydrolyzed, yielding putrescine.
- In the second pathway, arginine is converted into ornithine and then ornithine is converted into putrescine by ornithine decarboxylase (ODC).
- Putrescine can be converted into spermidine, spermine, and GABA. Putrescine can be converted to GABA by monoamino oxidases (MAO) and glutamylation then to succinate via transamination pathway.
- The polyamines, of which putrescine is one of the simplest, are growth factors necessary for cell division.
- Putrescine has specific role in skin physiology and neuroprotection, which include: a cation substitute, an osmolyte, or a transport protein.
- A steady supply of polyamines is a prerequisite for cell proliferation to occur.
- Polyamines, like Putrescine, play a role in spermatogenesis, skin physiology, promotion of tumorigenesis, organ hypertrophy and neuronal protection.
- Putrescine also plays a role in stress responses in plants, both to biotic and abiotic stressor and an important regulator in a variety of surface proteins, both on the cell surface and on organelles, such as the mitochondria and chloroplasts.
Melatonin
- Melatonin is a hormone released by the pineal gland at night, and plays a direct role in the control of the sleep-wake cycle by synchronizing circadian rhythms.
- Melatonin is a multifunctional indolamine compound and is synthesized from the metabolism of tryptophan via serotonin.
- In addition to being produced in the brain, melatonin is also produced in cells and tissues, such as the gastrointestinal system, gall, epithelial hair follicles, skin, retina, spleen, testis, salivary glands, bone marrow, leukocytes, placenta, and thrombocytes.
- Melatonin is a powerful antioxidant and anti-apoptotic agent.
- Melatonin prevents oxidative and nitrosative damage to all macromolecules due to its ability to form in metabolic activities, directly excrete toxic oxygen derivatives, and reduce the formation of reactive oxygen and nitrogen species.
- Melatonin hormone, which is a non-enzyme antioxidant, helps to eliminate harmful conditions stimulated by oxidative stress via inhibiting protein oxidation, lipid peroxidation, mitochondrial damage, and DNA degradation due to both its direct free radical scavenging activity and its contribution to the antioxidant defense system.
- Melatonin prevents linoleic acid, which is an energy source and growth factor of melatonin, which promotes the repair of damaged DNA, from entering the cell and suppresses its metabolism.
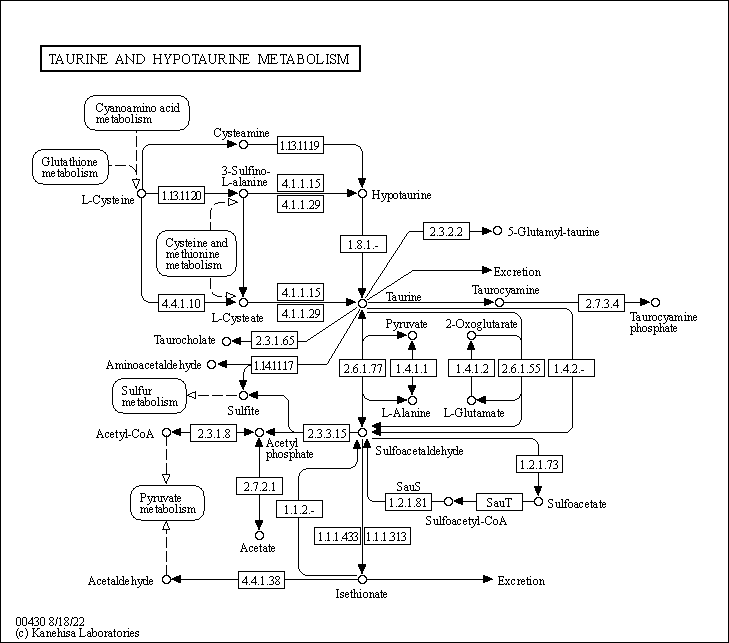
Taurine
- Taurine is a functional amino acid, found most abundantly distributed in mammalian tissues such as the brain, retina, heart, and skeletal muscle.
- Taurine was first isolated in the 1800s, but its first reported clinical use wasn’t until 1985, when heart failure patients in Japan were approved to receive taurine supplementation.
- Commonly seen marketed on energy drinks, a meta-analysis has shown that, under certain conditions, oral taurine supplementation can have a positive effect on athletic performance.
- Taurine is acquired through dietary uptake as well as the biosynthetic pathway of other sulfur-containing amino acids, methionine and cysteine.
- Taurine is a crucial metabolite in maintaining energy homeostasis: it plays a role in regulating the electron-transport chain and glutathione stores, as well as increasing membrane stability.
- Apart from its role in energy metabolism, taurine has been found to be an effective anti-oxidant by reducing free radicals and upregulating fatty acid oxidation.
- Taurine supplementation has been used to ameliorate the effects of cardiovascular diseases such as heart failure and hypertension, metabolic diseases like diabetes and obesity, as well as mitochondrial diseases such as mitochondrial myopathy, encephalopathy, lactic acidosis and stroke-like episodes (MELAS).
Urea
- Urea is the simplest amide of carbamic acid.
- Urea serves many processes, most notably nitrogen excretion. The liver forms urea by combining two ammonia molecules (NH3) with a carbon dioxide (CO2) molecule via the urea cycle.
- Ammonia is largely produced from the deamination of amino acids as the end-product of protein catabolism, along with aspartic acid from the urea cycle.
- Having no specific activity of its own, urea is an end, or waste, product. However, Urea is a biomarker for protein catabolism and functionality of the urea cycle. Urea is commonly linked to cancer and kidney disease.
- Urea-containing topical creams are used in the treatment of psoriasis, ichthyosis, atopic dermatitis, eczema, keratosis, keratoderma, and corns and calluses, as well as damaged and ingrown nails, and other dry, scaly conditions. Urea in skin care products helps to exfoliate and moisturize the skin.
- While urea is found in all cells, tissues and biofluids, abnormal levels have been associated with cirrhosis, cancer, uremia, infection and kidney function.
- A common blood test, the blood urea nitrogen (BUN) test reveals important information about how well your kidneys are working. A BUN test measures the amount of urea nitrogen that’s in your blood.
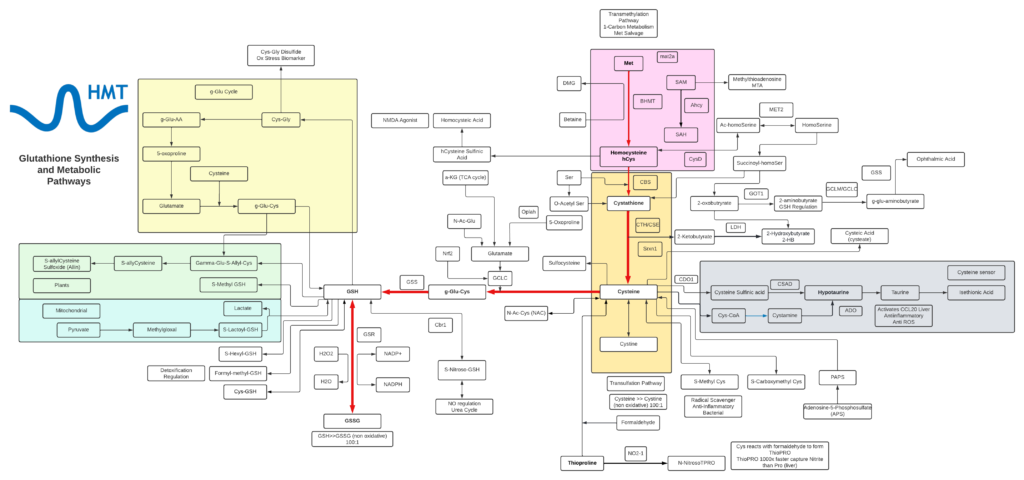
Glutathione
- Glutathione is a tripeptide comprised of cysteine, glycine, and glutamic acid and is found throughout most cells in the body.
- Cellular and mitochondrial levels of glutathione are highly associated with the health and longevity of individuals.
- Glutathione typically exists in cells in two states: reduced (GSH) and oxidized (GSSG). The ratio of these two states determines the redox status of a cell.
- GSSG can accumulate as a result of oxidative stress and can be directly toxic to cells and the depletion of glutathione triggers cell apoptosis.
- The critical roles of glutathione include: neutralization of free radicals, acts as a cofactor for several antioxidant enzymes, involved in the regeneration of vitamin C and E, transportation of mercury out of the cells and brain, key regulator of cellular proliferation and apoptosis, and vital to the function of mitochondria and maintaining mitochondrial DNA.
- GSH depletion is strongly associated with disease and loss of function with aging, some of which include: neurodegenerative disease, pulmonary disease, immune disease, liver disease, and cystic fibrosis.
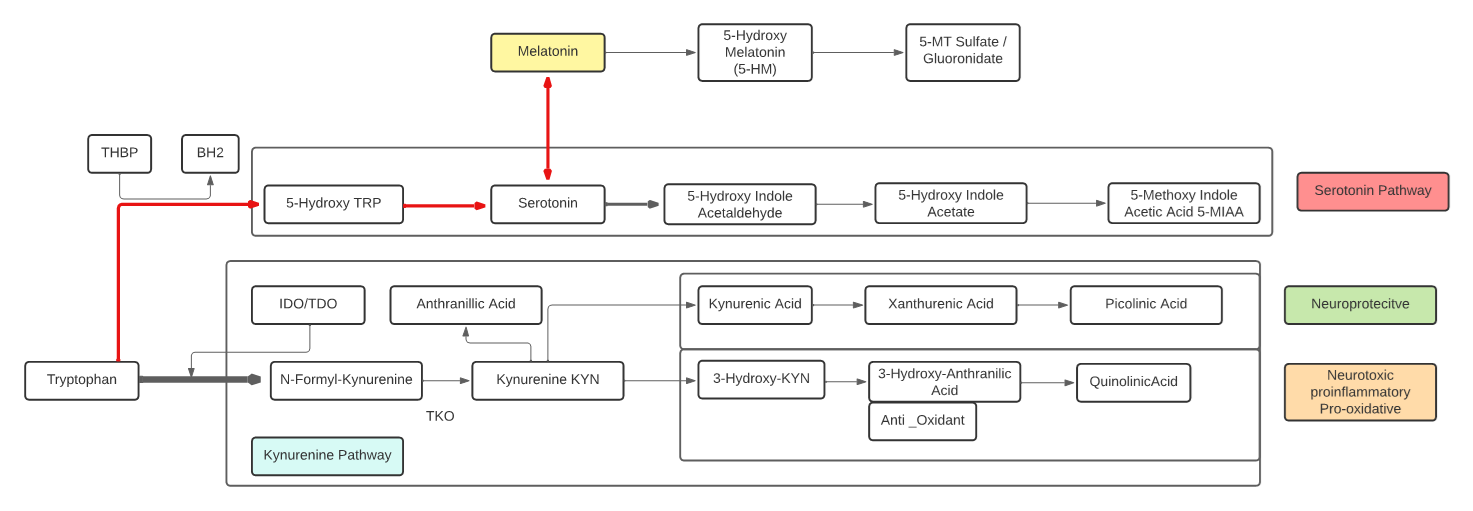
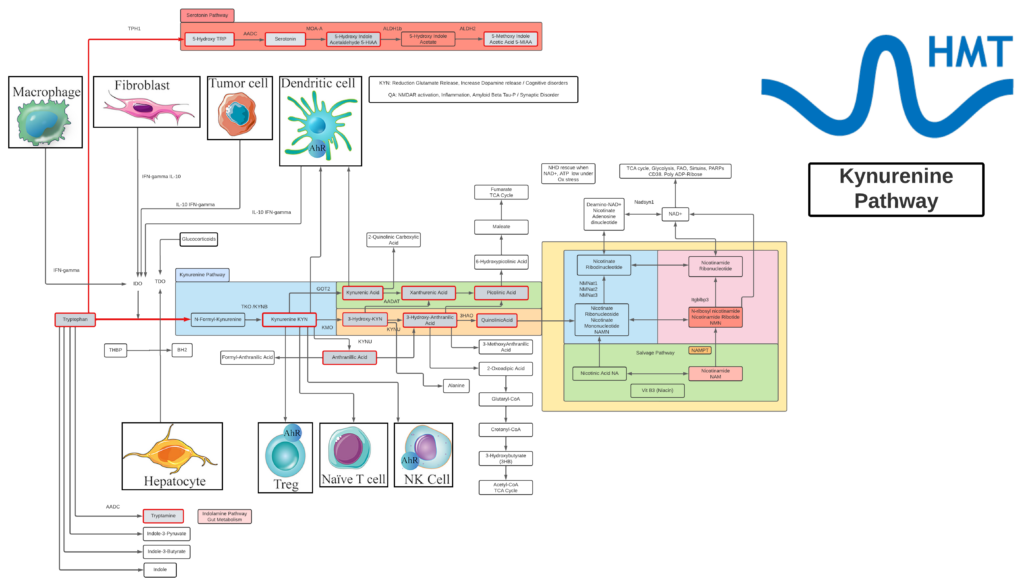
Kynurenine
- L-Kynurenine is a metabolite of the essential amino acid L-tryptophan.
- The majority of degradation of tryptophan is into Kynurenine (KYN) and the KYN pathway.
- Kynurenine is synthesized by the enzyme tryptophan dioxygenase (TDO), which is made primarily, but not exclusively in the liver, and indoleamine 2,3-dioxygenase (IDO), which is induced by interferon gamma (IFN-gamma or IFNG). IFNG is made in many tissues in response to immune activation.
- Kynurenine and its down-stream products carry out diverse biological functions, including dilating blood vessels during inflammation and regulating the immune response. Some cancers increase kynurenine production, which increases tumor growth.
- Kynurenine is the precursor to the neuroprotective metabolite, kynurenic acid (KA). Kynurenine is also the precursor to the neurotoxic metabolite, quinolinic acid (QA). Dysregulation in the balance between these two kynurenine metabolites has been observed in many disorders such as stroke, epilepsy, multiple sclerosis, and amyotrophic lateral sclerosis.
- Kynurenine activates the aryl hydrocarbon receptor (AhR), which plays a key role in immune suppression. AhR activation by kynurenine leads to a state of immunosuppression and serves to slow down immune response.
- Kynurenine may precipitate depressive symptoms associated with interferon treatment for hepatitis C. Cognitive deficits in schizophrenia are associated with imbalances in the enzymes that break down kynurenine. Blood levels of kynurenine are reduced in people with bipolar disorder. Kynurenine production is increased in Alzheimer’s and cardiovascular disease where its metabolites are associated with cognitive deficits and depressive symptoms.
- KYN degrades into two distinct pathways, when kynurenine-oxoglutarate transaminase KYN catabolizes its conversion into kynurenic acid (KA) and KA in turn degrades into Picolinic acid. Picolinic acid is produced under inflammatory conditions and a co-stimulus with interferon-gamma of macrophage effector functions. It is a selective inducer of the inflammatory protein-MIP-1alpha and -1beta, and there are two chemokines involved in the elicitation of the inflammatory reactions and in the development of Th1 responses.
- Alternatively, KYN degrades along the 3-hydroxykynurenine (3HK) pathway to Quinolinic Acid (QA). Quinolinic acid acts as an NMDA receptor agonist and has a possible role in neurodegenerative disorders. Within the brain, quinolinic acid is only produced by activated microglia and macrophages. While quinolinic acid cannot pass through the blood-brain barrier (BBB), kynurenic acid, tryptophan and 3-hydroxykynurenine can and act as precursors to the production of quinolinic acid in the brain.
- QA is an important metabolite as well for leading to the de novo synthesis of nicotinamide adenine dinucleotide (NAD+). As a precursor for NAD+, QA can direct a portion of tryptophan catabolism toward replenishing cellular NAD+ levels in response to inflammation and infection
NAD+
- Nicotinamide adenine dinucleotide (NAD+) is a molecule that sits at the crossroads of cellular and mitochondrial function, impinging on (directly and indirectly) metabolism, immune function, DNA repair and chromatin remodeling.
- The power of NAD+ is in its ability to participate in redox reactions, accepting a hydride ion and becoming NADH. NAD+/NADH ratios are commonly used in metabolomic studies to determine mitochondrial and cellular health.
- NAD+ is also used as cofactor or a substrate in hundreds of enzymatic processes, including poly-ADP-ribose polymerases (PARPs). Due to the high demand for NAD+, its levels are continuously sustained by nucleotide salvage pathways, as well as biosynthetic pathways, though this is restricted by cell type and availability of certain dietary nutrients.
- In perhaps the most important and conserved reaction in the living world: NADH generated by the TCA cycle is used to drive protons across the IMM through Complex I. This electrochemical potential is used, in turn, to generate ATP.
- Research has shown that levels of NADH decrease with age and disease, and targeting and restoring falling NAD+ levels has been shown to slow or even reverse disease progression
Dopamine
- Dopamine is a monoamine neurotransmitter that is made in your brain and acts as a chemical messenger. Its main function is communicating messages between nerve cells in your brain and the rest of your body.
- Dopamine plays a role in many body functions including movement, memory, reward and motivation, behavior and cognition, attention, sleep and arousal, mood, learning, and lactation.
- A dopamine molecule consists of a catechol structure with one amine group attached via an ethyl chain. Dopamine is the simplest possible catecholamine, a family that also includes the neurotransmitters norepinephrine and epinephrine. The presence of a benzene ring with this amine attachment makes it a substituted phenethylamine, a family that includes numerous psychoactive drugs.
- The direct precursor of dopamine, L-DOPA, can be synthesized indirectly from the essential amino acid phenylalanine or directly from the non-essential amino acid tyrosine. These amino acids are found in nearly every protein and readily available in food, with tyrosine being the most common. Although dopamine is also found in many types of food, it is incapable of crossing the blood–brain barrier that surrounds and protects the brain. It must therefore be synthesized inside the brain to perform its neuronal activity.
- The dopamine system plays a central role in several significant medical conditions, including Parkinson’s disease, attention deficit hyperactivity disorder, Tourette syndrome, schizophrenia, bipolar disorder, and addiction.
Asparagine
- Asparagine (asparagine, Asn) is an amino acid with a neutral side chain and is one of the building blocks of human proteins. It is widely distributed in plants, and the name asparagine is derived from its isolation from asparagus. Humans digest meat and fish proteins into amino acids and also absorb and utilize the asparagine contained in them.li>
- Asparagine is used as a food additive for nutritional support and other purposes. On the other hand, asparagine and reducing sugars in foods are thought to sometimes produce acrylamide upon heating and cooking.
- Asparagine is converted to aspartic acid by enzymes. Therefore, the actions of asparagine in vivo have much in common with those of aspartic acid. For example, it is thought to be effective in relieving fatigue because it provides a substrate for the citric acid circuit that produces energy. It is also thought to contribute to cell protection because it provides substrates for the urea circuit, which converts toxic ammonia produced within cells into less toxic urea.
- Asparagine is formed when an amino group is enzymatically transferred to aspartic acid. Aspartic acid is a nonessential amino acid because it is produced in the glycolytic and citric acid circuits, and its metabolite, asparagine, is also a nonessential amino acid. Asparagine is hydrolyzed to aspartate and ammonia by asparaginase. Aspartate then undergoes transamination to form glutamate and oxaloacetate from alpha-ketoglutarate. Oxaloacetate, which enters the citric acid cycle.
- The activity of asparagine and the enzymes involved in its utilization have also been implicated in cancer control and metastasis. For example, elevated expression of asparagine synthase and increased asparagine production contribute to cancer progression and metastasis, while L-asparaginase, which degrades asparagine, exhibits anticancer activity.
- Deamidation of asparagine (Asn) is also a nonenzymatic post-translational modification of proteins and free amino acid. Asn deamidation is associated with stored blood, pathogenesis of age-related diseases and hypofunction of monoclonal antibodies.
- Asparagine, colorectal cancer, and the role of sex, genes, microbes, and diet: A narrative review
https://www.frontiersin.org/articles/10.3389/fmolb.2022.958666/full. - Exploring the Role of Metabolites in Cancer and the Associated Nerve Crosstalk
https://www.mdpi.com/2072-6643/14/9/1722. - Asparagine in plants
https://onlinelibrary.wiley.com/doi/full/10.1111/j.1744-7348.2006.00104.x
bpspubs.onlinelibrary.wiley.com/doi/full/10.1038/bjp.2008.185.
Phenylalanine
- Phenylalanine (phenylalanine, Phe) is an amino acid with an aromatic benzyl group side chain and is one of the building blocks of human proteins. Humans digest meat and fish proteins into amino acids and absorb and utilize the phenylalanine contained in them.
- Phenylalanine is converted to tyrosine by enzymes, a material for the neurotransmitter’s dopamine, L-DOPA, noradrenaline, and adrenaline.
- Phenylalanine can also be converted (through a separate pathway) to phenylethylamine, a substance that occurs naturally in the brain and appears to elevate mood.
- Phenylalanine is an essential amino acid for humans. In plants and microorganisms, it is biosynthesized by the shikimic acid pathway, which starts with phosphoenolpyruvate in the glycolytic system and eritrose-4-phosphate in the pentose phosphate pathway.
- A genetic disease is known in which phenylalanine-4-monooxygenase, the enzyme that converts phenylalanine to tyrosine, is defective. In this disease, phenylalanine is not converted to tyrosine, resulting in the accumulation of phenylalanine and the production of another metabolite, phenylpyruvate.
- From phenylalanine, plants produce a variety of secondary metabolites. Vanillin, the aroma component of vanilla, cinnamaldehyde, the aroma component of cinnamon, and coumarin, which gives off a cherry blossom cake-like aroma, are biosynthesized from phenylalanine.
- Phenylalanine is both ketogenic and glucogenic. Phe is metabolized into acetoacetic acid and fumaric acid via tyrosine.
- Tyrosine, phenylalanine, and catecholamine synthesis and function in the brain
https://scholar.google.co.jp/scholar?output=instlink&q=info:ECdN6ZIrWDQJ:scholar.google.com/&hl=ja&as_sdt=0,5&as_rr=1&scillfp=10194320806884026631&oi=lle - Shikimate and phenylalanine biosynthesis in the green lineage
https://www.frontiersin.org/articles/10.3389/fpls.2013.00062/full. - Phenylketonuria: an inborn error of phenylalanine metabolism
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2423317/. - New insights into the shikimate and aromatic amino acids biosynthesis pathways in plants
https://scholar.google.co.jp/scholar?output=instlink&q=info:XpqzjXC7-YYJ:scholar.google.com/&hl=ja&as_sdt=0,5&as_rr=1&scillfp=9849204790047718433&oi=lle.
Metabolites come in many shapes and forms: polar and non-polar, basic, neutral and acidic. One of the challenges in the analysis of metabolomics data is understanding the roles of a given metabolite, considering the context of the particular experiment. The type of sample (tissue, blood, CSF, T cells, cancer cells etc.) has a lot to do with the role a metabolite may play. For most metabolites, there may be more than one pathway in which that metabolite is a product or reactant, and whose concentration may be dependent upon the context of the experiment, epigenetic and environmental factors, and other variables.
While a metabolite’s reactivity (its role as a reactant or product) in a specific biochemical reaction within a specific pathway may be of importance in one case, the physical properties and/or accumulation of that metabolite may be its importance in another (e.g. accumulation leading to acidosis, its role as an osmoprotectant or a hydrator or an emollient).
The HMT library is not about explaining metabolite structure. Instead, the HMT metabolite data base is focused on the most common pathways and biology around each metabolite. The focus is on mammalian biology, however, in many cases plant and bacterial pathways can also be relevant. We like to think that there is still much to learn about how metabolite levels are influenced and look to learn more.
We will start with amino acids and some amino acid derivatives then move to selected organic acids. Each week a new metabolite will appear. We hope you enjoy this addition to our website. If so, you can subscribe to our newsletter or contact us for more information on how we can help you get the most of out of metabolomics – no matter your field or interests.